What Is The Oxidation Number Of Elements In Group 1
2 1 2 0. All the elements of group 17 form compound in odd oxidation states 1 1 3 5 7 but down the group importance of the higher oxidation states generally decreases.
Oxidation Number Periodic Table Elements Priyamstudycentre
Those with oxidation number 1 are in group 17 the halogens f cl br i at.
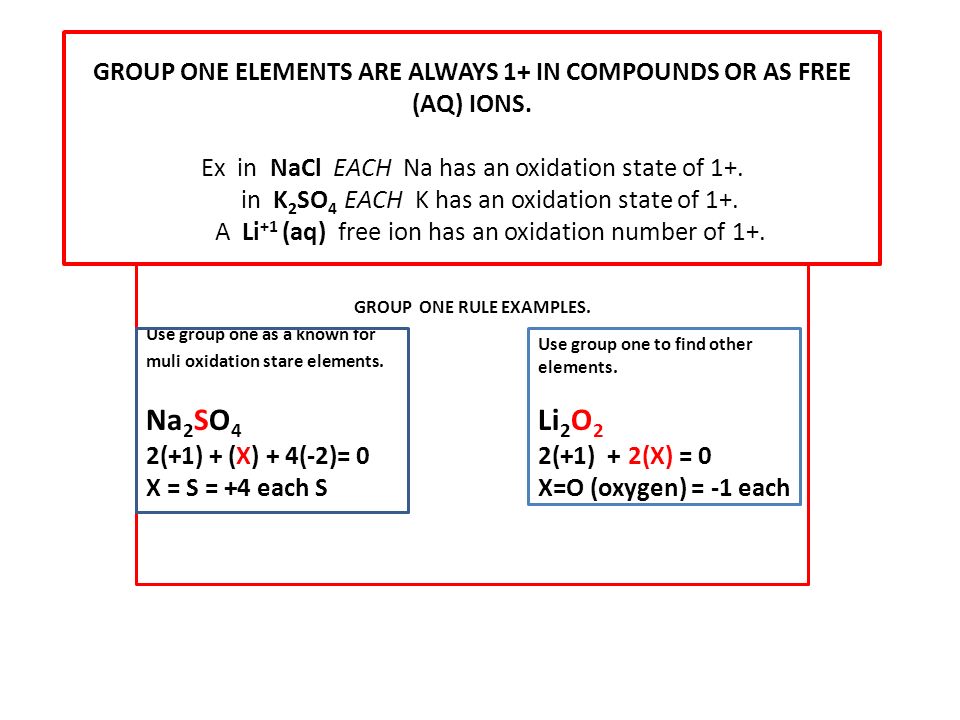
What is the oxidation number of elements in group 1. When hydrogen forms compounds with metals hydrogen s oxidation number is 1. Oxygen has an oxidation of 2 in most of its compounds. The oxidation state of hydrogen in a compound is usually 1.
Alternatively you can think of it that the sum of the oxidation states in a neutral compound is zero. Hydrogen forms three oxidation states 1 0 1. Nah and cah 2 are some examples.
All alkali metals group 1 elements have an oxidation state of 1 in their compounds. The elements with oxidation number of 1 are those is group 1 of the periodic table h li na k rb cs and fr. Typically this relates to the number of electrons that must be gained negative oxidation number or lost positive oxidation number for the atom s valence electron shell to be filled or half filled.
The oxidation number of fluorine is always 1. This is an. The oxidation state of oxygen is usually 2 except in compounds with fluorine oxygen has a positive oxidation number.
The elements in group viia often form compounds such as alf 3 hcl and znbr 2 in which the nonmetal has a 1 oxidation number. However in the case of peroxides the oxidation number corresponding to oxygen is 1. The oxidation state sometimes referred to as oxidation number describes the degree of oxidation loss of electrons of an atom in a chemical compound conceptually the oxidation state which may be positive negative or zero is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100 ionic with no covalent component.
The elements with oxidation number of 1 are those is group 1 of the periodic table h li na k rb cs and fr. Peroxides include hydrogen peroxide h 2 o 2. Oxidation numbers of group 1 group 2 elements are studied.
The sum of the oxidation numbers in a polyatomic ion is equal to the charge on the ion. However most metals are capable of multiple oxidation states. Chlorine bromine and iodine usually have an oxidation number of 1 unless they re in combination with an oxygen or fluorine.
Those with oxidation number 1 are in group 17 the halogens f cl br i at. Since group 1 metals always have an oxidation state of 1 in their compounds it follows that the hydrogen must have an oxidation state of 1 1 1 0. The oxidation number of the.
However when bonded with an element with less electronegativity than it it exhibits an oxidation number of 1. Oxidation number 0 occurs only in hydrogen molecule 1 oxidation state examples. The oxidation number refers to the electrical charge of an atom.
The sum of the oxidation numbers in a neutral compound is zero. If the hydrogen is part of a binary metal hydride compound of hydrogen and some metal then the oxidation state of hydrogen is 1.
Solved Determine The Oxidation Number Oxidation State O
8 Redox Biogeochemistry Land Use And Water Quality
Periodic Table Of The Elements Oxidation Numbers
Ppt Oxidation Numbers Powerpoint Presentation Free Download
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctvqd6ovrriiuhcw6mdf3ctjhygnqep5i2yka6b3frypepxwgsj Usqp Cau
What Is The Oxidation Number Of A Group Viia Element In A Compound
Reactions Of Main Group Elements With Hydrogen Chemistry Libretexts
Unit 11 Review For Reactivity Name 1 What Kinds Of Molecules And
Explain Me This Calculating Oxidation States
Oxidation Number Definition Rules Examples Video Lesson
Simplified Chemistry Concepts Chemistry P Block Elements Simply
Main Group Elements Definition
Calculating The Oxidation State Of A Carbon Master Organic Chemistry
How To Find Oxidation Number Oxidation State Determination Jee
Elements Of S Block Properties Of The First Group Elements 1a
Oxidation States Introduction To Chemistry
Oxidation State Trends In Periodic Table Video Khan Academy
Redox Reactions Solutions Examples Activities Experiment Videos
Oxidation State Trends In Periodic Table Video Khan Academy
Oxidation State And Problems Ejercicios De Biotecnologia Docsity
Oxidation State Examples Online Chemistry Tutor
5 What Are The Oxidation States For A Elements In Group 1 B
How To Know The Oxidation Numbers Of All The Elements By Group
How To Calculate Oxidation Numbers Introduction Youtube
How To Find Oxidation Numbers 12 Steps With Pictures Wikihow
Oxidation State Examples Online Chemistry Tutor
Group Periodic Table Wikipedia
The Periodic Table Of Oxidation States Compound Interest
Chemical Forums B Family Of Periodic Table
Cesium Description Symbol Uses Facts Britannica
Oxidation States Of Transition Metals Video Lesson Transcript
Rules Of Oxidation Number Assignment Ppt Video Online Download
Oxidation Numbers An Oxidation Number Is The Charge Or The
How Do You Calculate The Oxidation Number Of An Element In A
Posting Komentar
Posting Komentar