Ionic Bonds Are Formed When
Ions are another name for charged atoms. An ionic or electrovalent bond is formed between two ions of opposite charges.
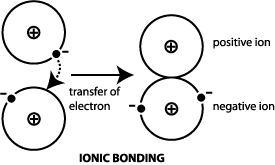
Such a bond forms when the valence outermost electrons of one atom are transferred permanently to another atom.

Ionic bonds are formed when. Ionic bond is the type of bond in which electrons can be transferred from one atom to another leading to the formation of positive and negative ions. In a true covalent bond the electronegativity values are the same e g h 2 o 3 although in practice the electronegativity values just need to be close if the electron is shared equally between the atoms forming a covalent bond then the bond is said to be nonpolar usually an electron is more attracted to one atom than to. Some elements are electropositive and some are electronegative.
Learn more about ionic bonds in this article. The elements take on either a negative or positive charge. Each atom consists of protons neutrons and electrons.
This is possible only if one of the atoms loses one or more electrons and the other gains. An ionic bond forms between two ions of opposite charges. To better understand why and how ions atoms that have a charge due to the loss or gain of electrons are formed you can study what happens during the chemical reaction to create salt.
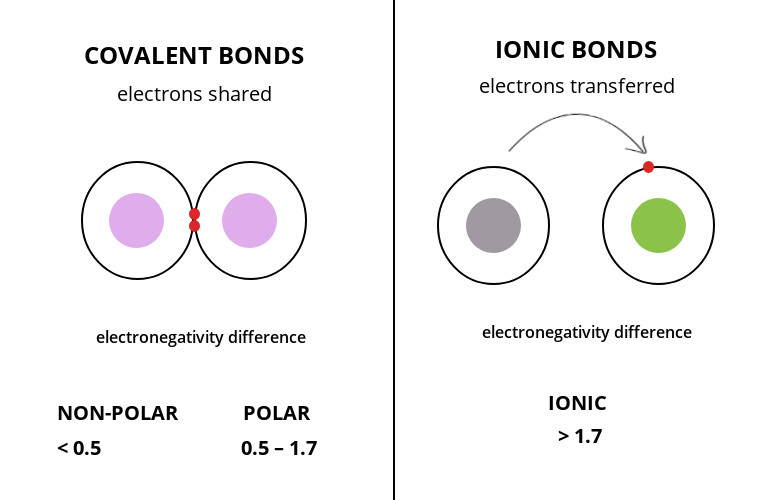
In ionic bonding electrons transfer from one atom to another. In a covalent bond the atoms are bound by shared electrons. Ionic bonds are also formed when there is a large electronegativity difference between two atoms.
The electrostatic attraction between positive and negative ions holds the compounds together. Ionic bond type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. During the formation of an ionic bond one of the reacting elements should form a positively charged ion cation and the other should give a negatively charged ion anion.
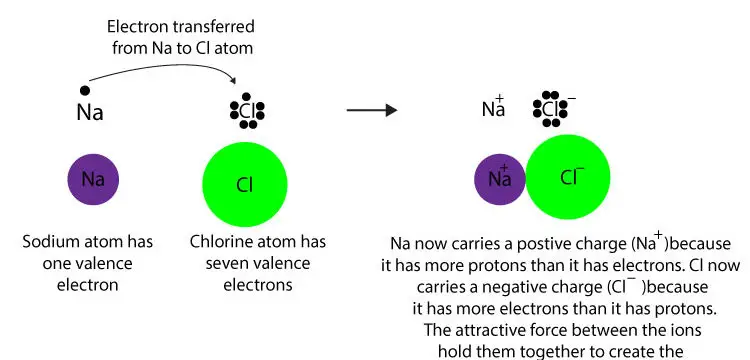
This difference causes an unequal sharing of electrons such that one atom completely loses one or more electrons and the other atom gains one or more electrons such as in the creation of an ionic bond between a metal atom sodium and a nonmetal. About covalent and ionic bonds. Why and how ions are formed ionic bonding is the type of bonding that holds salts together.
Forming ionic bonds positive and negative ions form when a metal reacts with a non metal by transferring electrons. Atoms that gain electrons make negatively charged ions called. It is also known as an electrovalent bond and is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical.
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions and is the primary interaction occurring in ionic compounds it is one of the main types of bonding along with covalent bonding and metallic bonding ions are atoms or groups of atoms with an electrostatic charge. The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the sharing is so unequal that an electron from atom a is completely lost to atom b resulting in a pair of ions. The oppositely charged ions are strongly attracted to each other forming.
 Electronegativity Bond Scale Surfguppy Chemistry Made Easy
Electronegativity Bond Scale Surfguppy Chemistry Made Easy
 Learning Objectives Ionic Bonding Describe How Ions Are Formed
Learning Objectives Ionic Bonding Describe How Ions Are Formed
 Explain The Formation Of Ionic Bonds With Examples A Plus Topper
Explain The Formation Of Ionic Bonds With Examples A Plus Topper
 Ces Information Guide Materials Science Engineering
Ces Information Guide Materials Science Engineering
 Ppt 7 1 Formation Of Ionic Bonds Donating And Accepting
Ppt 7 1 Formation Of Ionic Bonds Donating And Accepting
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcs2rvy6nhvonwri1k70okkgi2avo Pczlc9jm1 F9oaxzryqqft Usqp Cau
Molecules And Compounds Overview Atomic Structure Article
 Ionic Bonds In Chemistry Ionic Bond Examples With Explanation
Ionic Bonds In Chemistry Ionic Bond Examples With Explanation
 7 Ionic Bonding 7 1 Formation Of Ionic Bonds Donating And
7 Ionic Bonding 7 1 Formation Of Ionic Bonds Donating And
 Explain The Formation Of Ionic Bonds With Examples A Plus Topper
Explain The Formation Of Ionic Bonds With Examples A Plus Topper
How Does The Formation Of An Ionic Bond Differ From That Of A

 Formation Of Ionic Bond Ck 12 Foundation
Formation Of Ionic Bond Ck 12 Foundation
 What Is An Ionic Bond Simple Science Chemistry Quatr Us Study
What Is An Ionic Bond Simple Science Chemistry Quatr Us Study
Powerschool Learning 8th Grade Science Sec 2 Ionic Bonds
 Covalent Bonds Vs Ionic Bonds Difference And Comparison Diffen
Covalent Bonds Vs Ionic Bonds Difference And Comparison Diffen
 Chapter 7 Ionic Bonding Ppt Download
Chapter 7 Ionic Bonding Ppt Download
7 Ionic Bonding Chemistry Batz
 Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrstvynt Eit Cc7tecy7os8fok1blbpprsza Usqp Cau
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrstvynt Eit Cc7tecy7os8fok1blbpprsza Usqp Cau
 Bonding Chemistry Gcse Revision
Bonding Chemistry Gcse Revision
 How Do Atoms Form An Ionic Bond
How Do Atoms Form An Ionic Bond
 What Are Ionic Compounds And How They Are Formed
What Are Ionic Compounds And How They Are Formed
What Is The Ionic Bond Formation Sodium Chloride Socratic
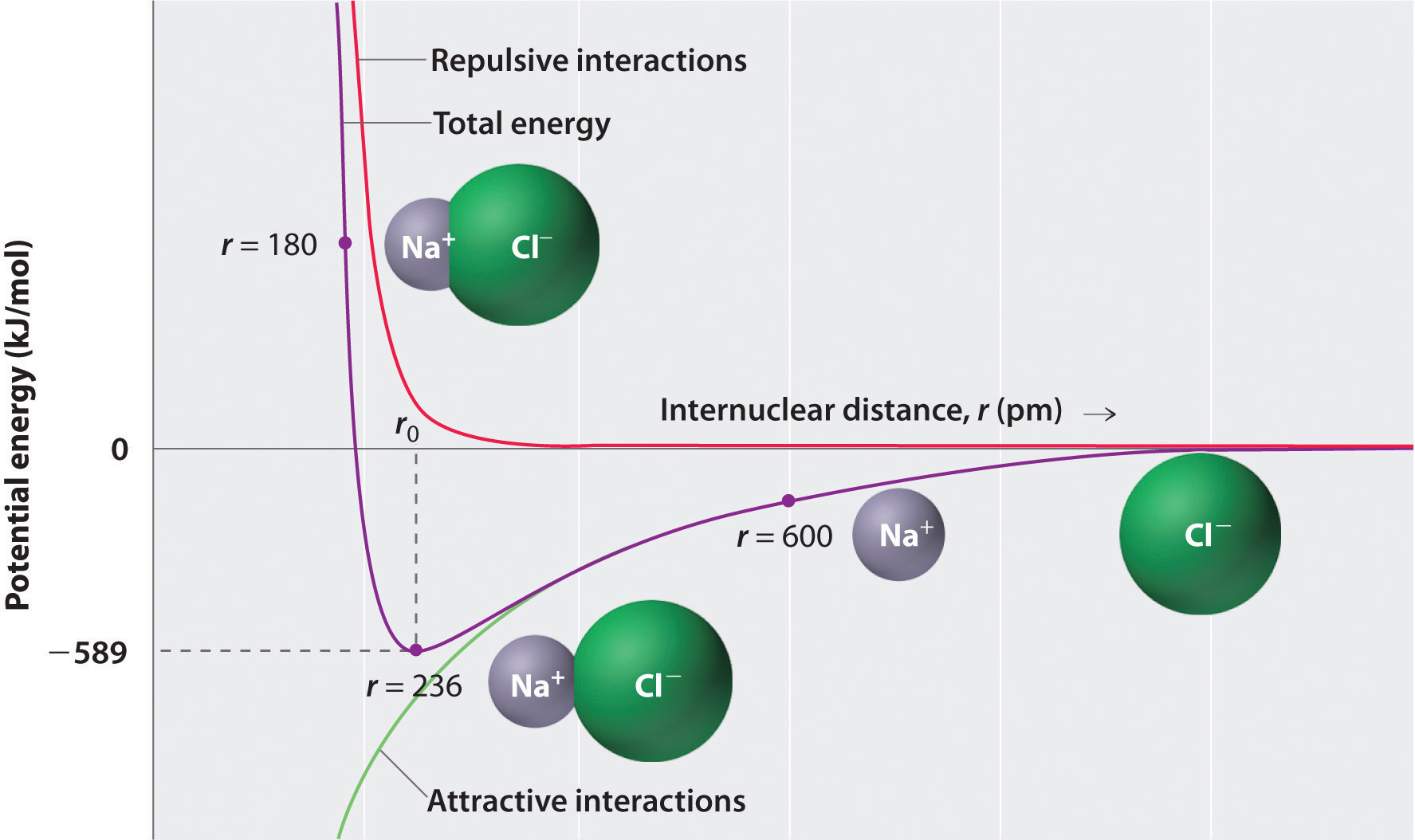 8 2 Ionic Bonding Chemistry Libretexts
8 2 Ionic Bonding Chemistry Libretexts
 Dublin Schools Lesson Ionic Bonds How Do Ionic Compounds Form
Dublin Schools Lesson Ionic Bonds How Do Ionic Compounds Form
Examples Of Ionic Bonds And Compounds
 Ionic Bond Definition Properties Examples Facts Britannica
Ionic Bond Definition Properties Examples Facts Britannica
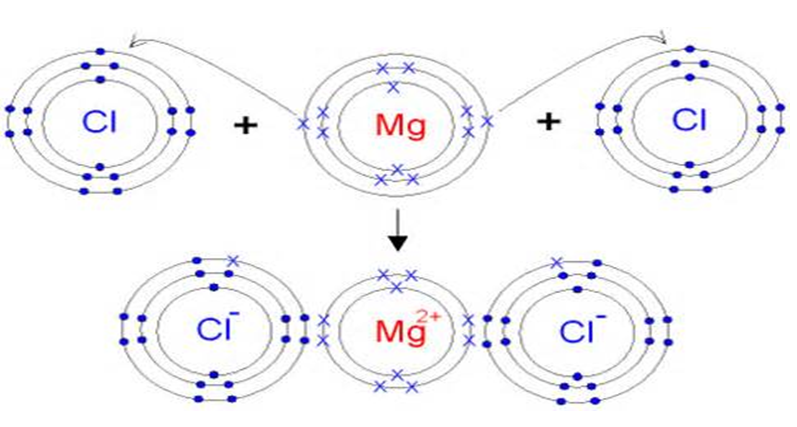 What Are Ionic Compounds Definition Structure Properties
What Are Ionic Compounds Definition Structure Properties
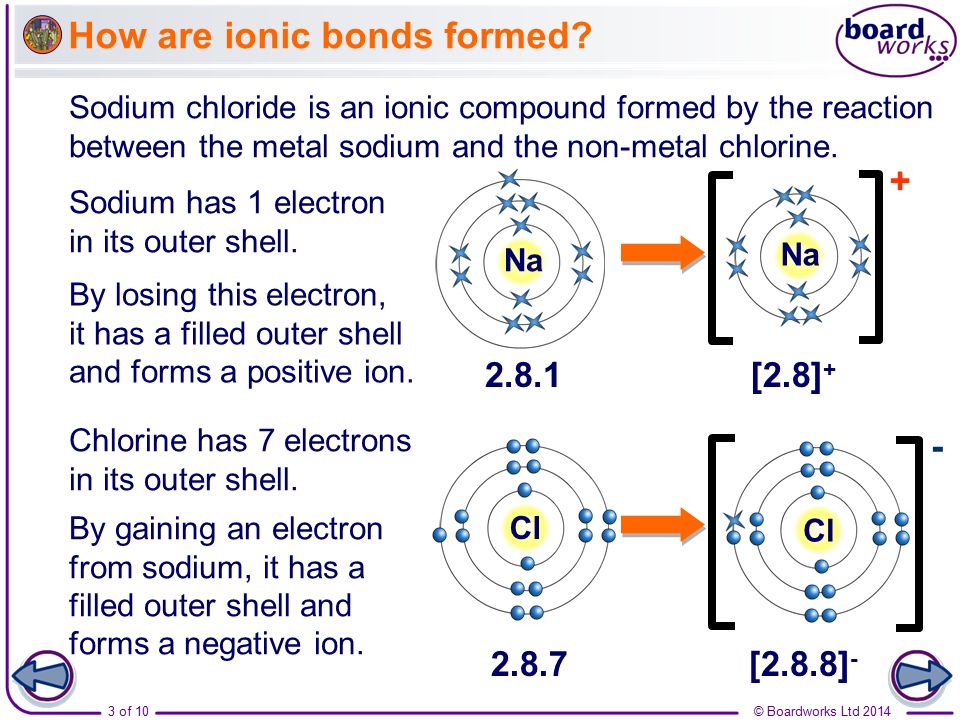 Boardworks Gcse Additional Science Chemistry Ionic Bonding Ppt
Boardworks Gcse Additional Science Chemistry Ionic Bonding Ppt
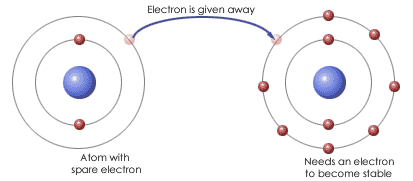 Ionic Bond Ellesmere Chemistry Wiki Fandom
Ionic Bond Ellesmere Chemistry Wiki Fandom
 Explain The Formation Of Ionic Bonds With Examples A Plus Topper
Explain The Formation Of Ionic Bonds With Examples A Plus Topper
 Chemsolve Net Ionic Bond Definition Example Properties And
Chemsolve Net Ionic Bond Definition Example Properties And
 Factors Influencing The Formation Of Ionic Bonds Video Lesson
Factors Influencing The Formation Of Ionic Bonds Video Lesson


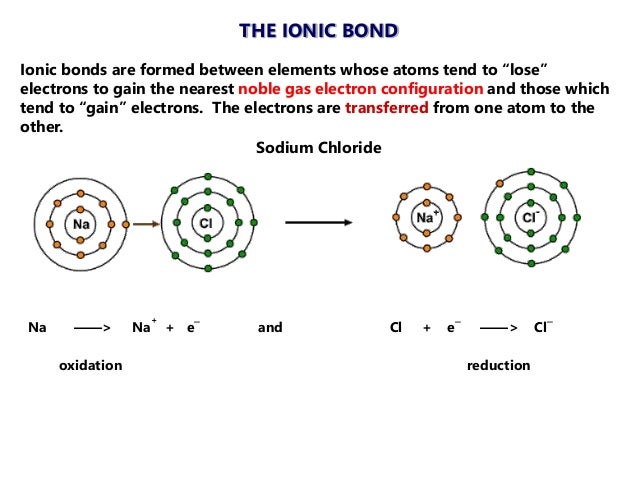
Posting Komentar
Posting Komentar