How Do Buffers Work
It is important to know that a ph buffer is a substance that resists a change in ph when small amounts of. Buffers in the human body.
A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base.

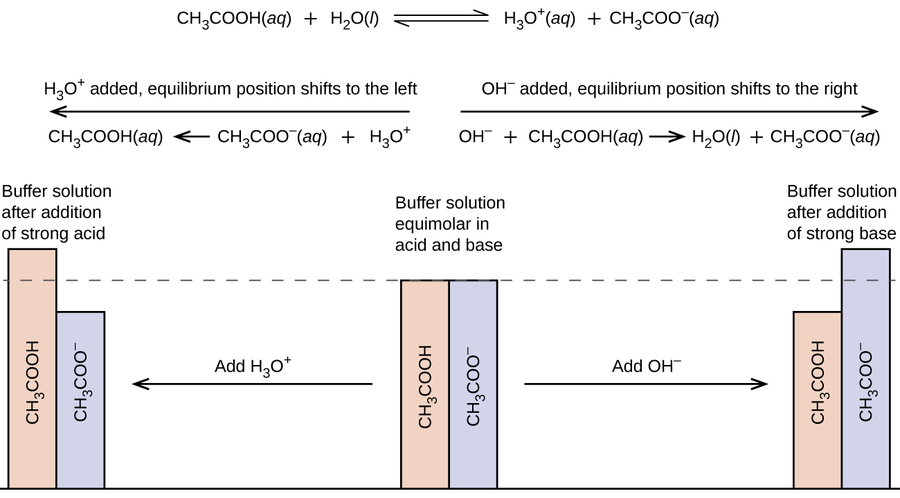
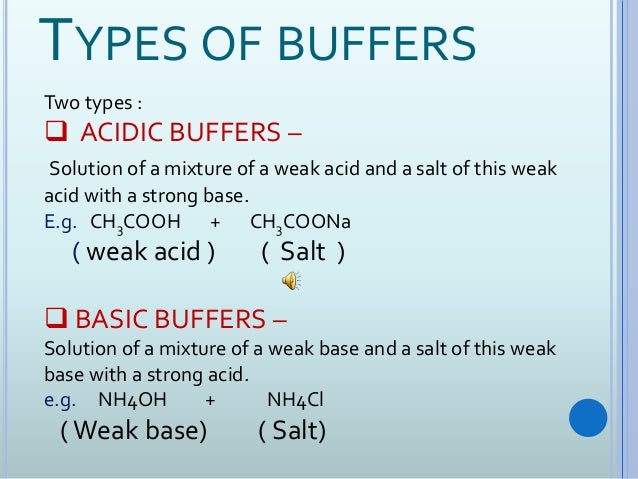
How do buffers work. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. We ll take a mixture of ethanoic acid and sodium ethanoate as typical. It can be also defined as the quantity of strong acid or base that must be added to change the ph of one liter of solution by one ph unit.
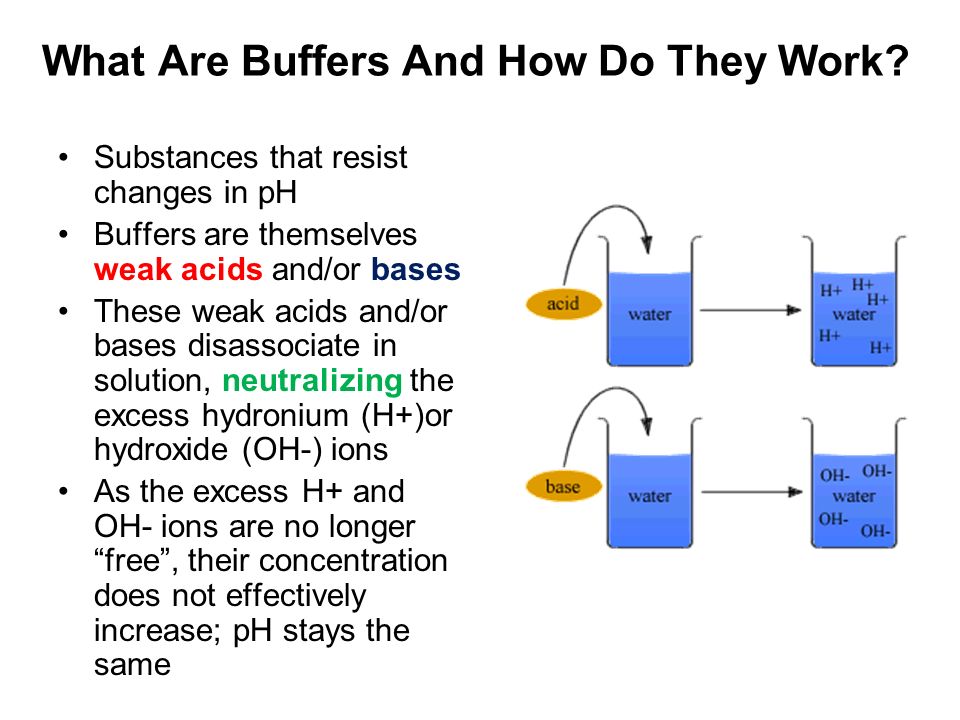
It is able to neutralize small amounts of added acid or base thus maintaining the ph of the solution relatively stable. How do ph buffers work. However the buffer allows the ph to not change.
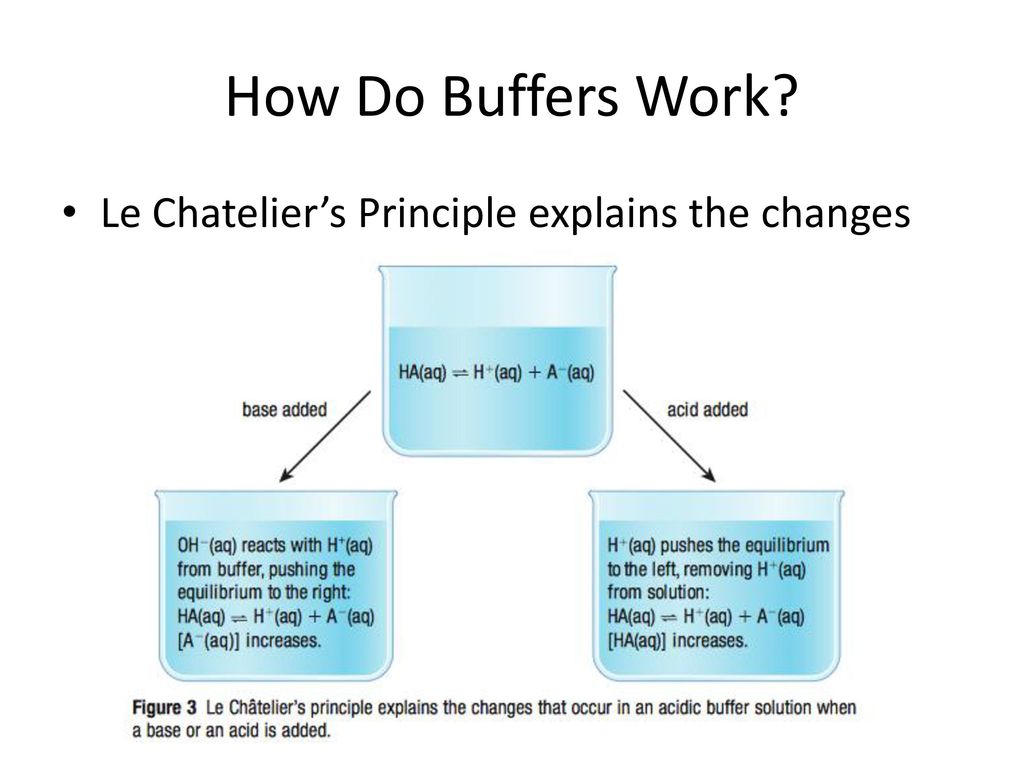
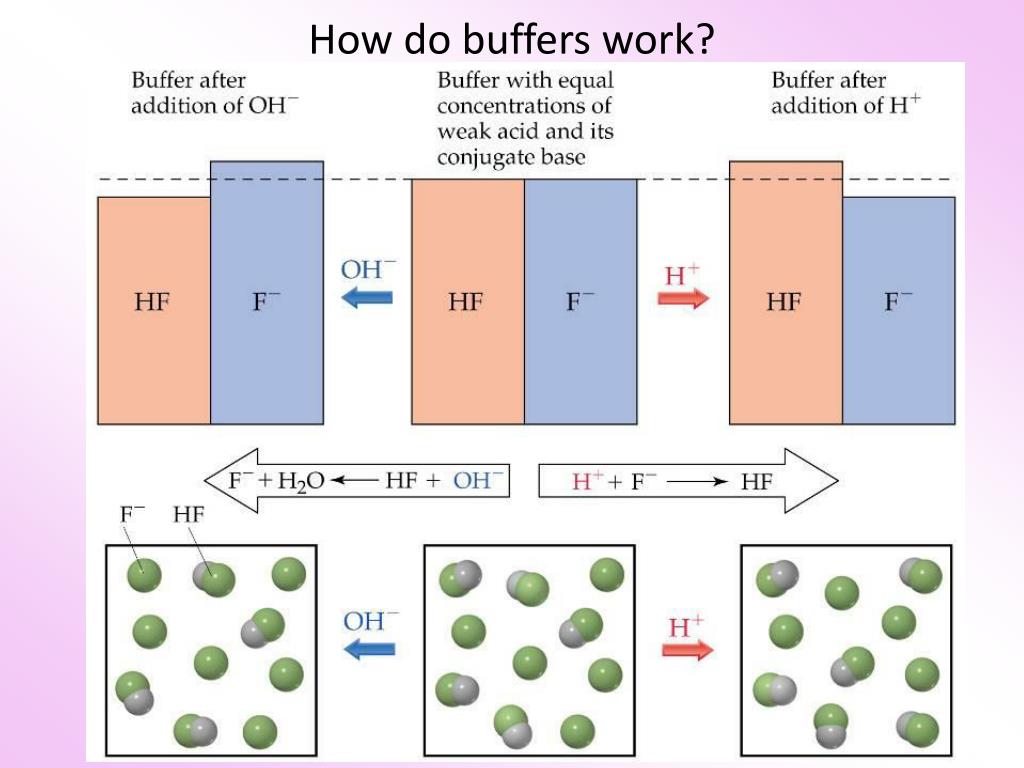
A buffer is a special solution that stops massive changes in ph levels. A buffer solution has to contain things which will remove any hydrogen ions or hydroxide ions that you might add to it otherwise the ph will change. Acidic and alkaline buffer solutions achieve this in different ways.
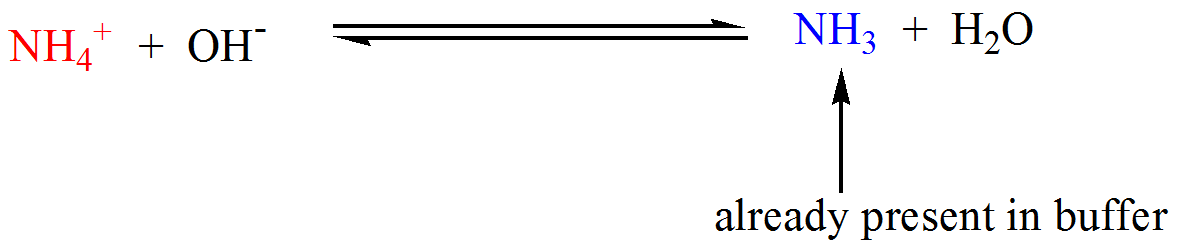
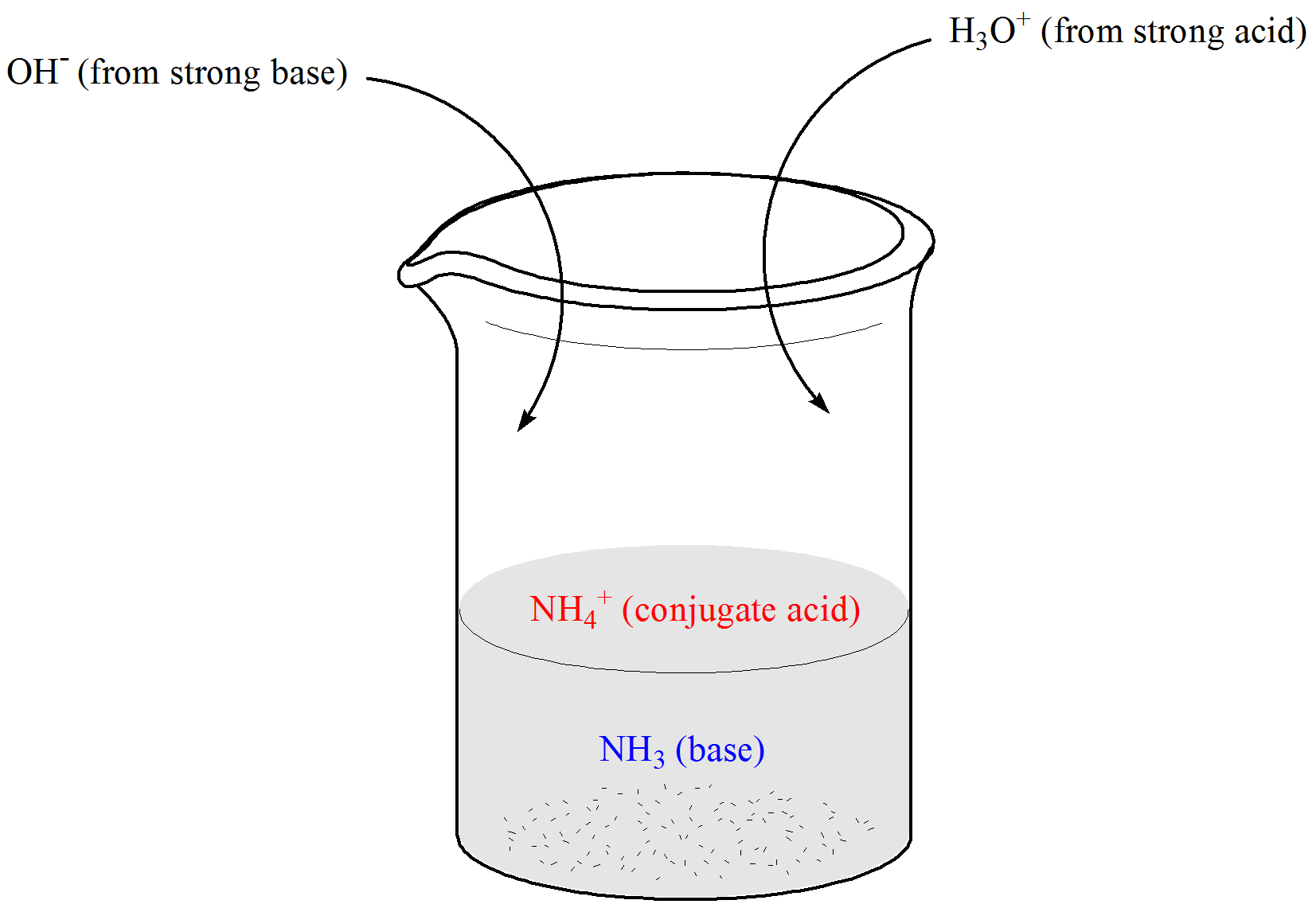
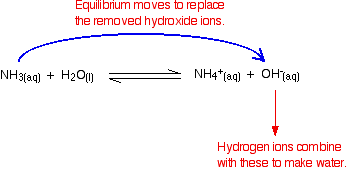
Let s take an example of a buffer made up of the weak base ammonia nh3 and its conjugate acid nh4. A buffer is able to not change in the ph because of the pair of conjugate base and conjugate acid which are in the buffer. Every buffer that is made has a certain buffer capacity and buffer range.
H a ha. Buffers work by reacting with any added acid or base to control the ph. How do buffers work.
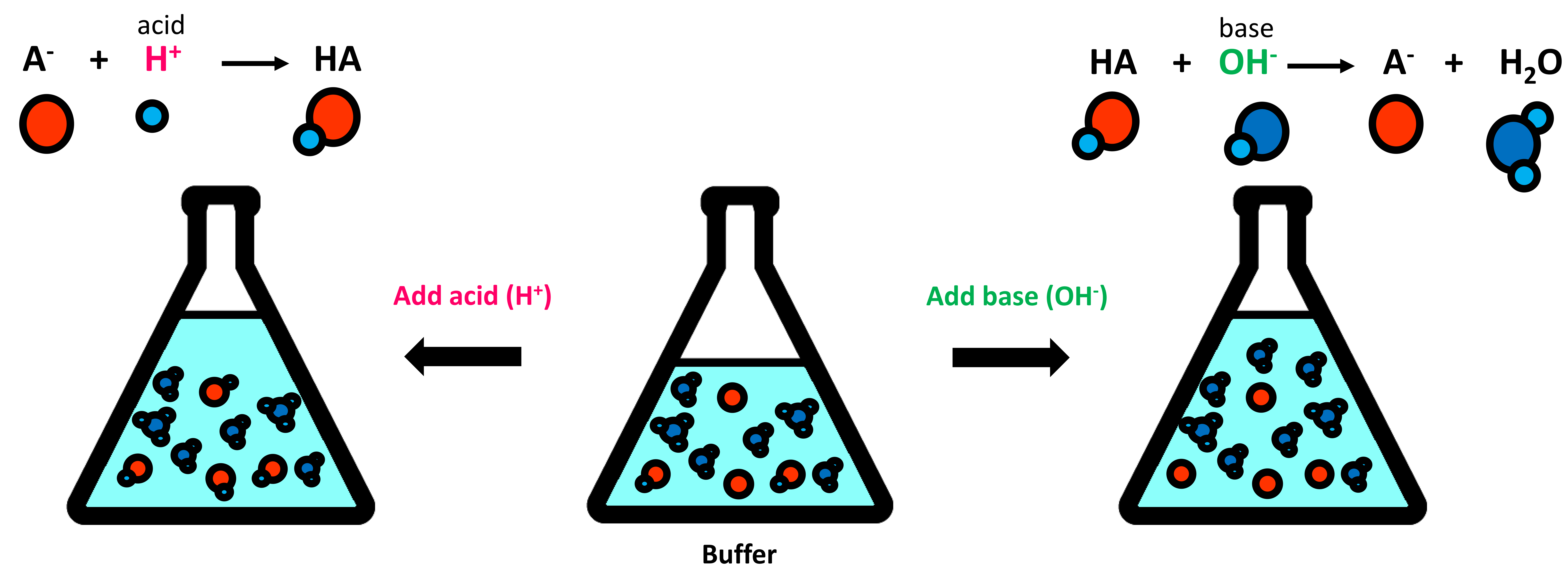
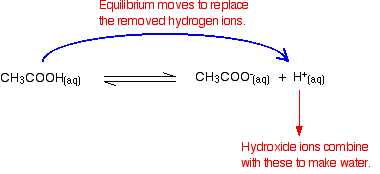
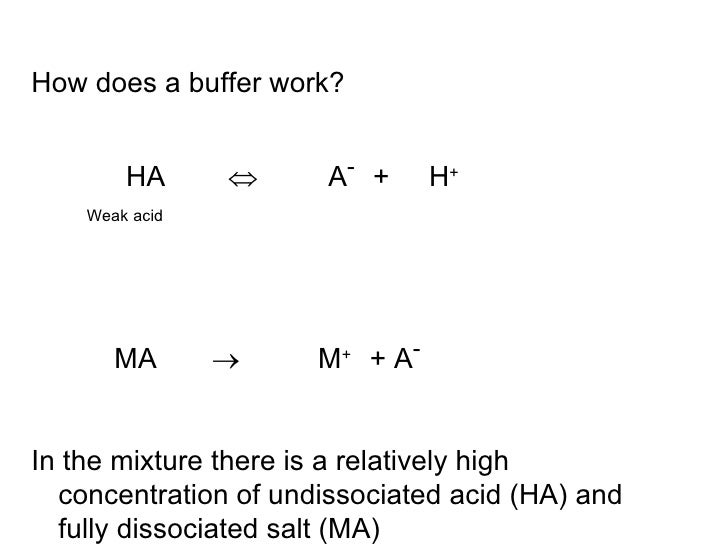
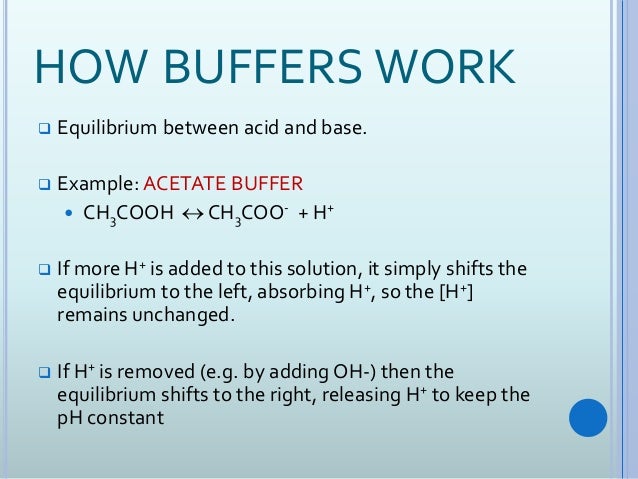
Buffers work by neutralizing any added acid h ions or base oh ions to maintain the moderate ph making them a weaker acid or base. The h gets absorbed by the a instead of reacting with water to form h 3 o h so the ph changes only slightly. The buffer capacity is the amount of acid or base that can be added before the ph begins to change significantly.
The ph is high or low depending on how much of the acid and base there is. A buffer absorbs or releases free floating hydrogens out of into a solution to maintain the ph of that solution from what does a buffer release hydrogens. For example let s consider the action of a buffer composed of the weak base ammonia nh 3 and its conjugate acid nh 4.
Importance to the body. A buffer is simply a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is an aqueous solution that has a highly stable ph.
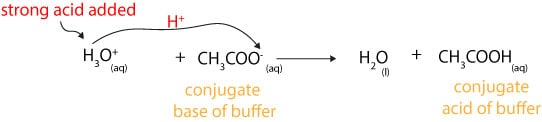
This is important for processes and or reactions which require specific and stable ph ranges. When a ph buffer reacts with an acid molecules in the buffer bind to the loose hydrogen molecules. If a strong acid is added to a buffer the weak base will react with the h from the strong acid to form the weak acid ha.
How do buffer solutions work. There are two key terms associated with buffers. Similarly adding water to a buffer or allowing water to evaporate will not change the ph of a buffer.
How do buffers work. If you add an acid or a base to a buffered solution its ph will not change significantly. When hcl strong acid is added to this buffer system the extra h ions added to the system are consumed by the nh3 to form nh4.
 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctrmtg1 8sxrz Thmxkqnmmlmlfy3nqccay V5qtxhndd5nqbiw Usqp Cau
 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
 Chapter 8 8 Buffer Systems Ppt Download
Chapter 8 8 Buffer Systems Ppt Download
 What Is A Biological Buffer And How To Choose The Best Buffer For
What Is A Biological Buffer And How To Choose The Best Buffer For
 Title Lesson 9 Buffers Ppt Video Online Download
Title Lesson 9 Buffers Ppt Video Online Download
 Michel Lotito 6 15 50 6 25 07 Ppt Download
Michel Lotito 6 15 50 6 25 07 Ppt Download
 Buffer System In Chemistry Definition Overview Video Lesson
Buffer System In Chemistry Definition Overview Video Lesson
 Acid Base Equilibria Common Ions Consider Solution Containing Hf
Acid Base Equilibria Common Ions Consider Solution Containing Hf
 How Does A Buffer Solution Work Youtube
How Does A Buffer Solution Work Youtube
Buffers Indicators Acids And Bases 101 The Basics Of Chemistry
 What Is A Buffer And How Does It Work Westlab
What Is A Buffer And How Does It Work Westlab
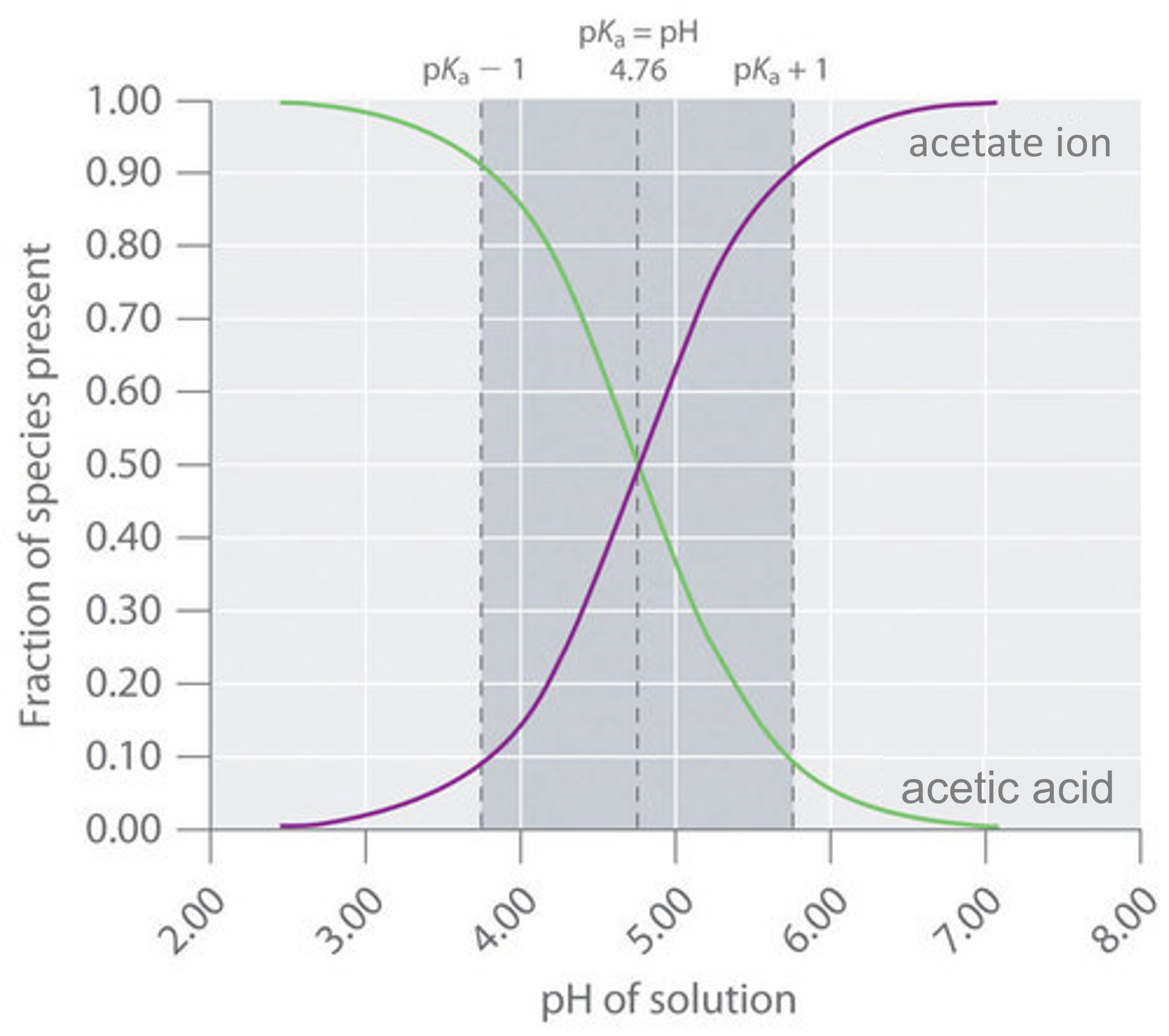 8 8 Buffers Solutions That Resist Ph Change Chemistry Libretexts
8 8 Buffers Solutions That Resist Ph Change Chemistry Libretexts
 Buffer System In Chemistry Definition Overview Video Lesson
Buffer System In Chemistry Definition Overview Video Lesson
 How Does A Buffer Maintain Ph Chemistry Libretexts
How Does A Buffer Maintain Ph Chemistry Libretexts
 Buffer Calculator Sigma Aldrich
Buffer Calculator Sigma Aldrich
 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
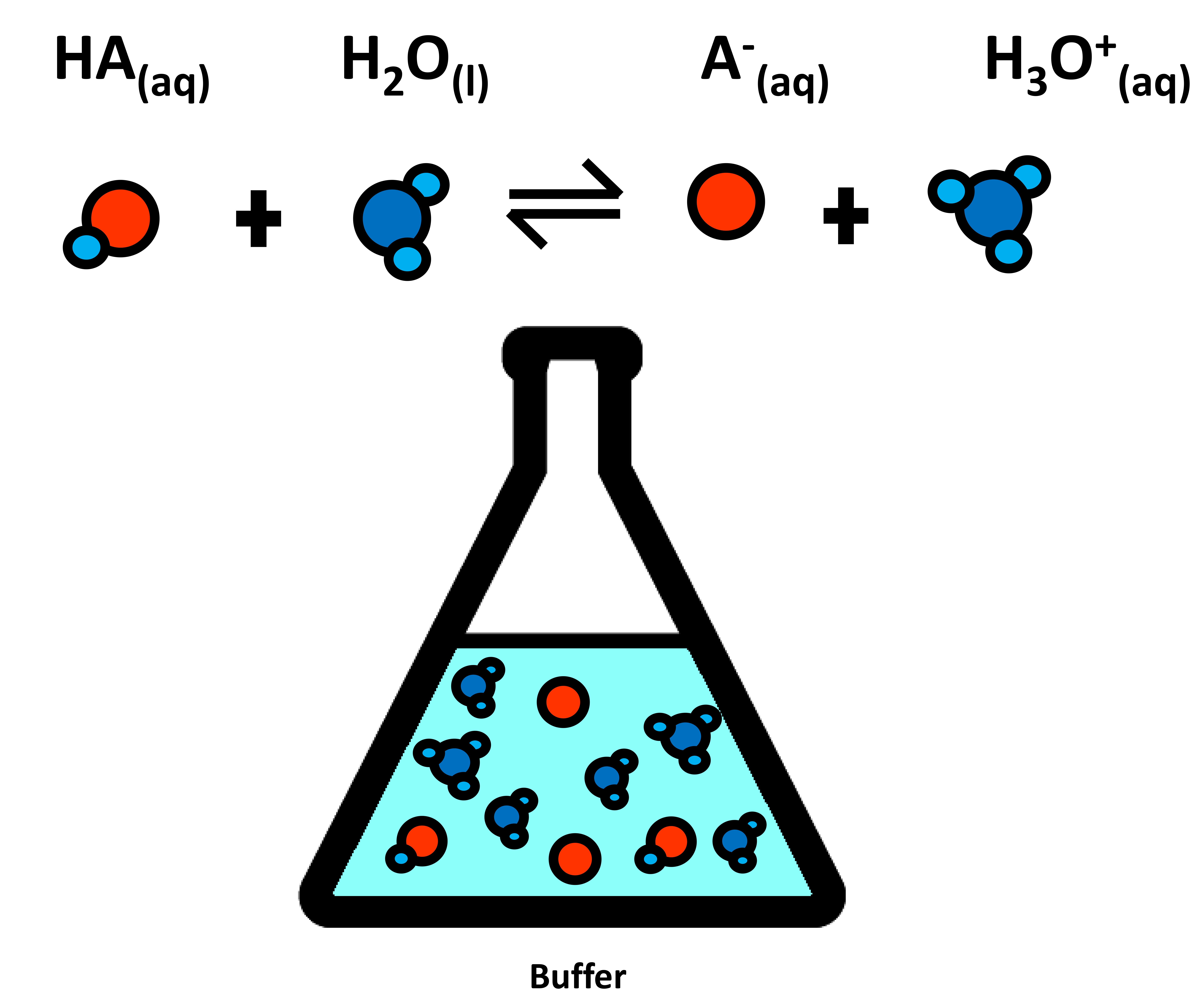 What Is A Biological Buffer And How To Choose The Best Buffer For
What Is A Biological Buffer And How To Choose The Best Buffer For
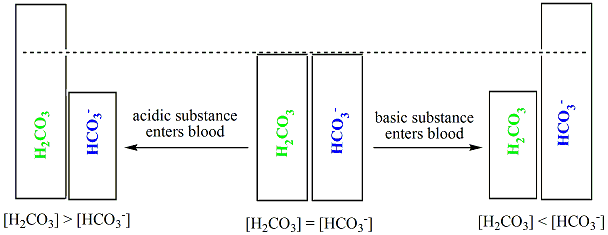 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Upper Secondary Ydp Student Activity How Do
 Solved 1 Define The Term Common Ion Effect Used In The
Solved 1 Define The Term Common Ion Effect Used In The
 How Are Buffer Solutions Used Example
How Are Buffer Solutions Used Example
 Buffer Solution What Is It And How Does It Work To Resist Changes
Buffer Solution What Is It And How Does It Work To Resist Changes
 Ppt Chemistry Chapter 15 Applications Of Aqueous Equilibria
Ppt Chemistry Chapter 15 Applications Of Aqueous Equilibria
 7 1 Acid Base Buffers Chemistry Libretexts
7 1 Acid Base Buffers Chemistry Libretexts
 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy



Posting Komentar
Posting Komentar