What Is Hess S Law Equation
This law is a manifestation that enthalpy is a state function. Hess lawallows the enthalpy change δh for a reaction to be calculated even when it cannot be measured directly.
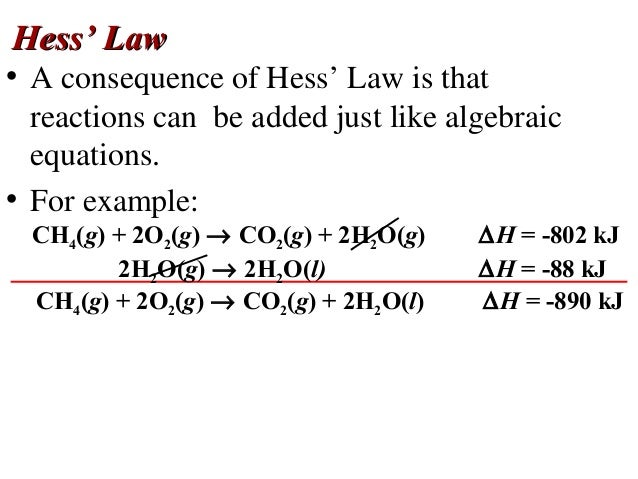
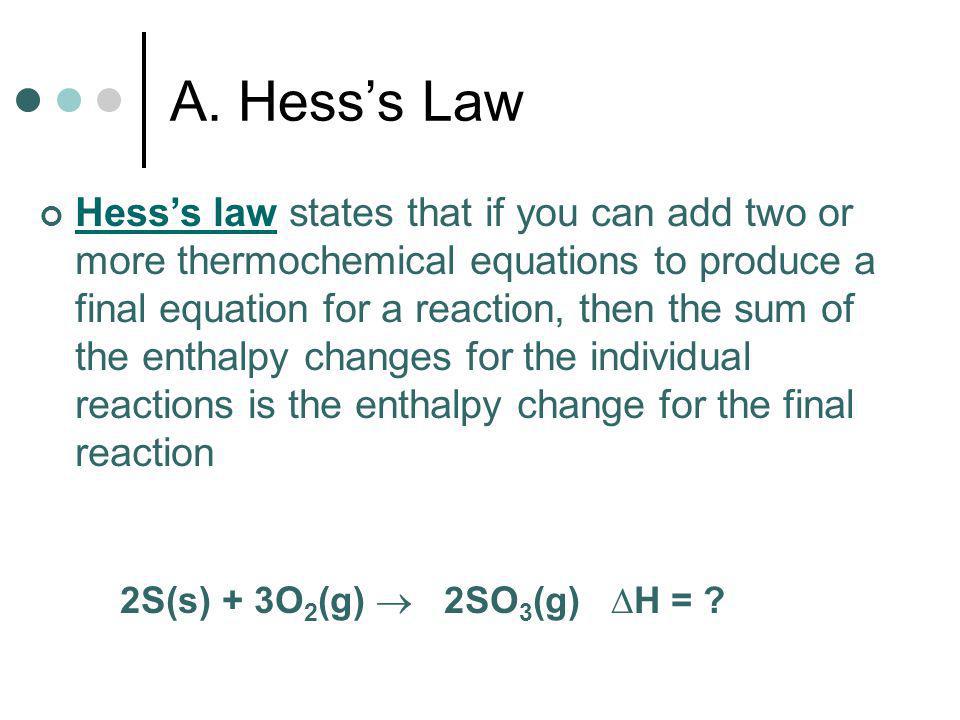 Thermochemistry Chapter 17 4 Hess S Law And Ppt Video Online
Thermochemistry Chapter 17 4 Hess S Law And Ppt Video Online
Updated december 03 2019 hess s law also known as hess s law of constant heat summation states that the total enthalpy of a chemical reaction is the sum of the enthalpy changes for the steps of the reaction.
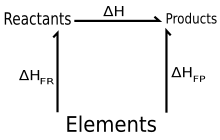
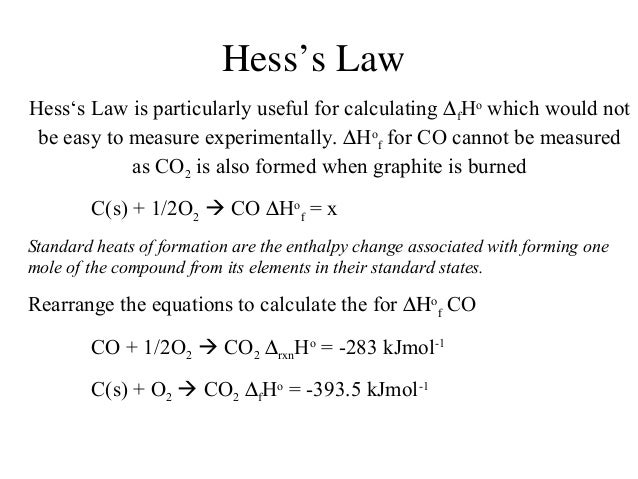
What is hess s law equation. This is accomplished by performing basic algebraic operations based on the chemical equation of reactions using previously determined values for the enthalpies of formation. A brief discussion about how hess law is used followed by some examples. If it occurs in two steps the enthalpy changes of these two steps are δh 2 and δh 3.
This is known as hess s law and is given in the following equation. This is achieved by carrying out simple algebraic operations based on the chemical equation of reactions using values previously defined for the formation enthalpies. So the reaction can happen in one step where the enthalpy change is δh 1.
Another way to state hess law is. Hess s law states that no matter the multiple steps or intermediates in a reaction the total enthalpy change is equal to the sum of each individual reaction. A representation of hess law where h represents enthalpy hess law of constant heat summation also known as hess law or hess s law is a relationship in physical chemistry named after germain hess a switzerland born russian chemist and physician who published it in 1840.
Hess s law allows the enthalpy shift even if it can not be determined directly to be estimated for a reaction. The enthalpy change for the overall process is the sum of the enthalpy change of the steps in the process. According to hess s law if a reacts to form the product b it doesn t matter how many steps involved to get the product the total enthalpy change will be same.
δ h r x n δ h 1 δ h 2 δ h 3. It is also known as the conservation. The law states that the total enthalpy change during the complete course of a chemical reaction is the same whether the reaction is made in one step or in several steps.
Hess s law of constant heat summation or just hess s law states that regardless of the multiple stages or steps of a reaction the total enthalpy change for the reaction is the sum of all changes. Therefore you can find enthalpy change by breaking a reaction into component steps that have known enthalpy values. If a chemical equation can be written as the sum of several other chemical equations the enthalpy change of the first chemical equation equals the sum of the enthalpy changes of the other chemical equations.
 Hess S Law The Algebraic Addition Of Chemical Equations Yields A
Hess S Law The Algebraic Addition Of Chemical Equations Yields A
 Hess S Law Quiz Quiz Questions Answers Online Personality Test
Hess S Law Quiz Quiz Questions Answers Online Personality Test
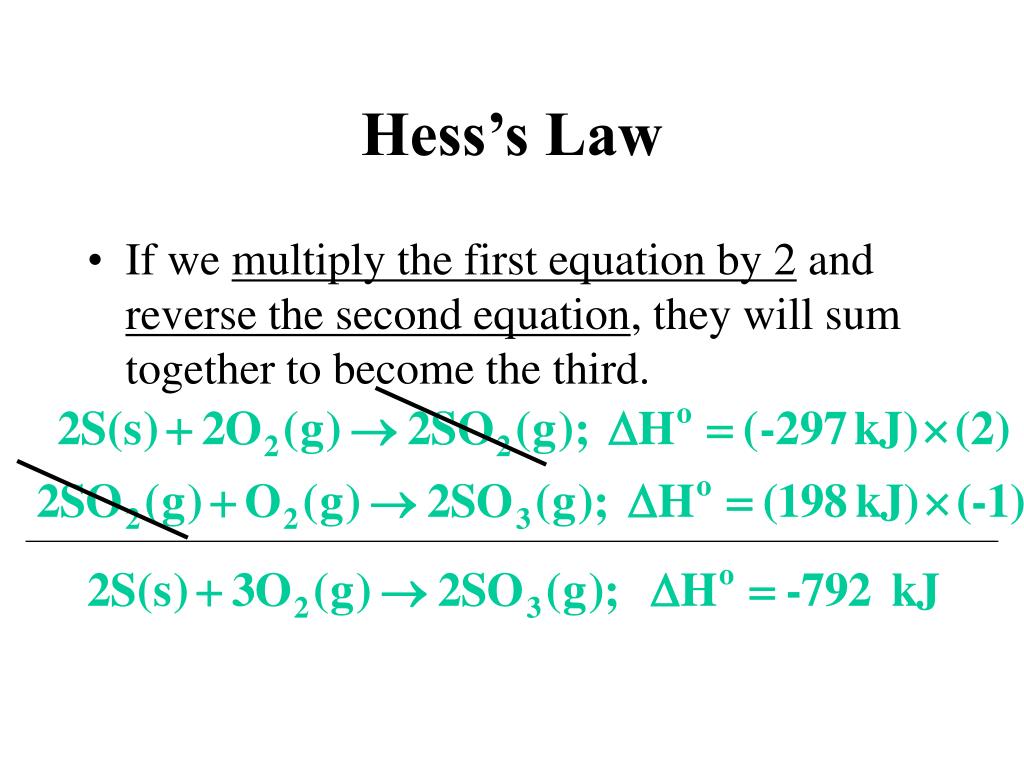 Ppt Hess S Law Powerpoint Presentation Free Download Id 456765
Ppt Hess S Law Powerpoint Presentation Free Download Id 456765
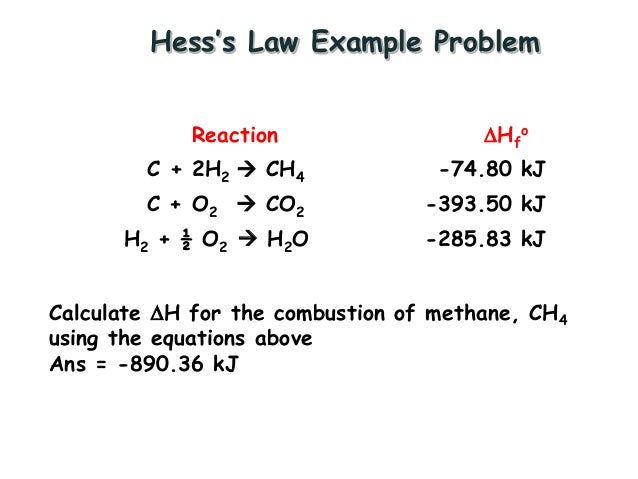
 Hess S Law Example Youtube Chemistry Health Science Hess
Hess S Law Example Youtube Chemistry Health Science Hess
 Hess S Law Example Video Enthalpy Khan Academy
Hess S Law Example Video Enthalpy Khan Academy
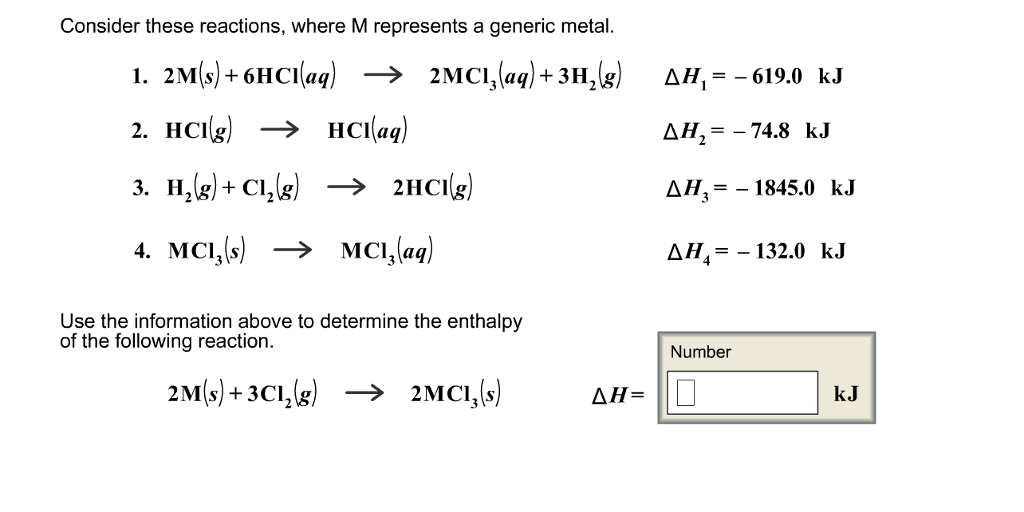 Solved Does This Problem Involve Hess S Law That Law Is
Solved Does This Problem Involve Hess S Law That Law Is
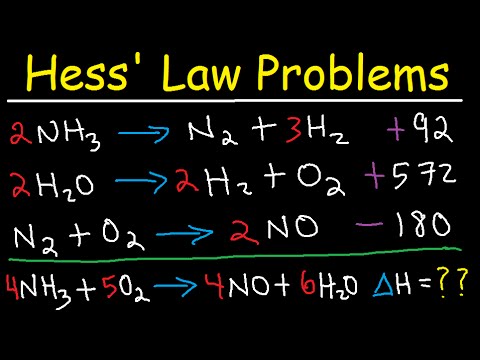 Hess Law Chemistry Problems Enthalpy Change Constant Heat Of
Hess Law Chemistry Problems Enthalpy Change Constant Heat Of
 Solved Using Hess S Law And Equations Below Calculate De
Solved Using Hess S Law And Equations Below Calculate De
 5 2 Hess S Law Ib Chemistry Ellesmere College
5 2 Hess S Law Ib Chemistry Ellesmere College
 Hess S Law Of Heat Summation Chemistry For Non Majors
Hess S Law Of Heat Summation Chemistry For Non Majors
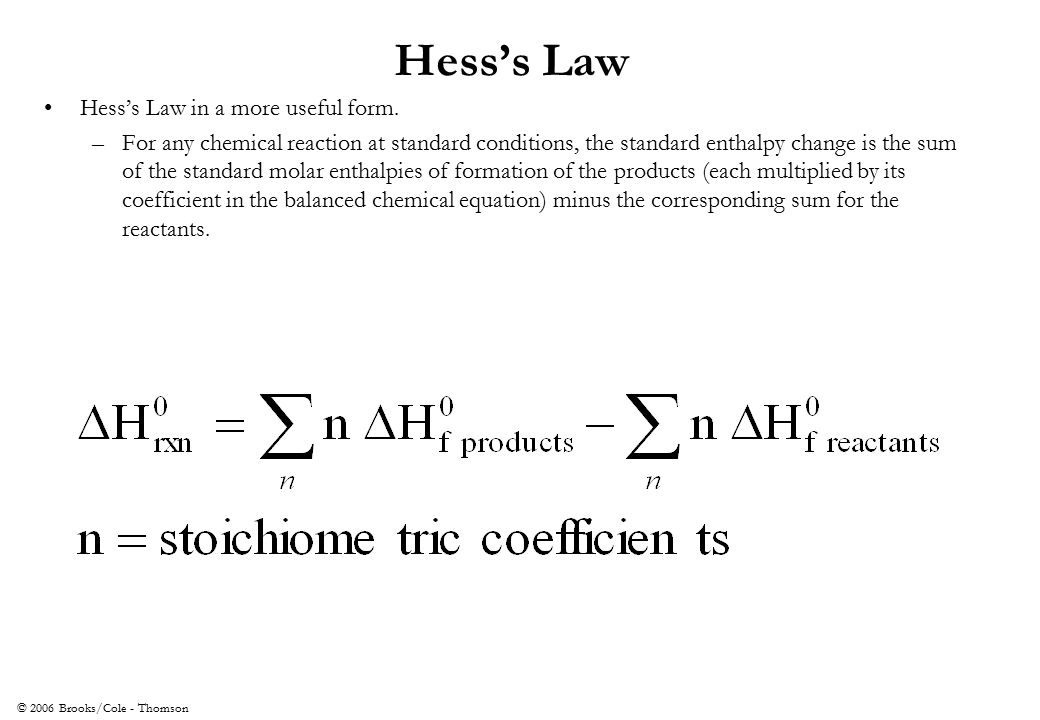
 Hess S Law Definition Formula Examples Video Lesson
Hess S Law Definition Formula Examples Video Lesson
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrosxmlpguhatwtogxw1rw2wo179msvibvu5iip9z7ghrcqjhve Usqp Cau
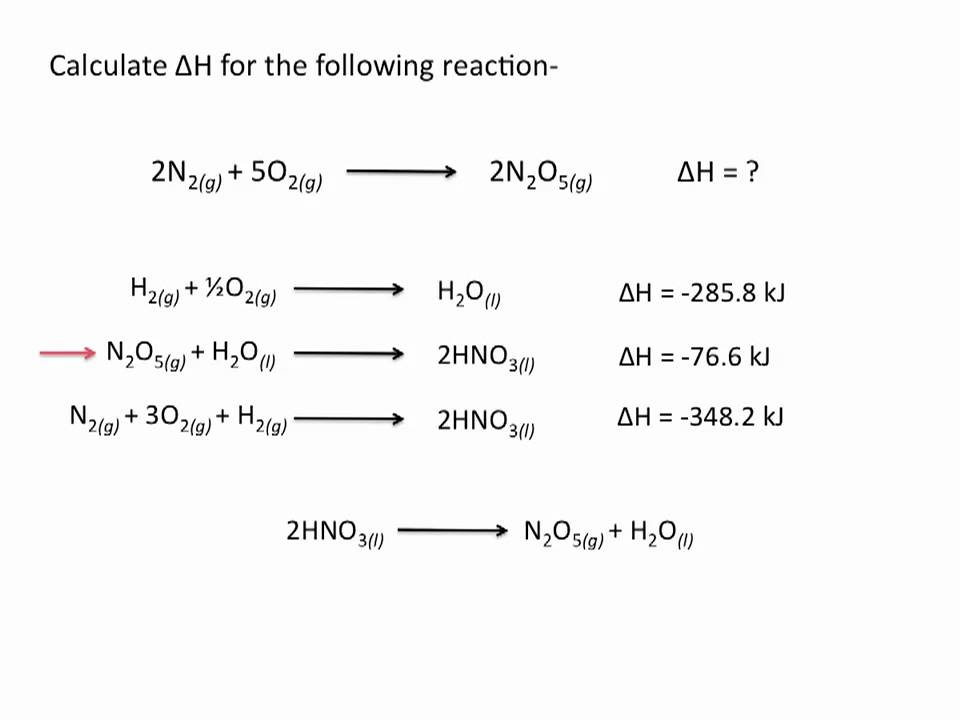 Hess S Law Chemistry Tutorial Youtube
Hess S Law Chemistry Tutorial Youtube
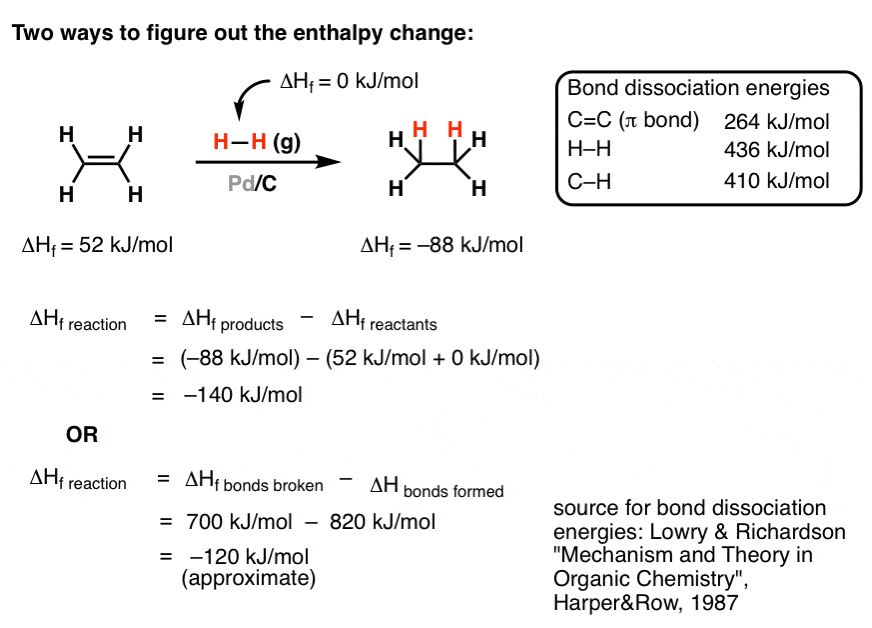 From Gen Chem To Organic Chem Pt 10 Hess Law Master Organic
From Gen Chem To Organic Chem Pt 10 Hess Law Master Organic
Hess S Law Introduction To Chemistry
 Energy Lecture 4 Hess S Law Review Ppt Download
Energy Lecture 4 Hess S Law Review Ppt Download
 Ppt Hess S Law Powerpoint Presentation Free Download Id 6193634
Ppt Hess S Law Powerpoint Presentation Free Download Id 6193634
What Is Hess S Law Of Heat Summation Example
 Hess S Law Definition Formula Examples Video Lesson
Hess S Law Definition Formula Examples Video Lesson
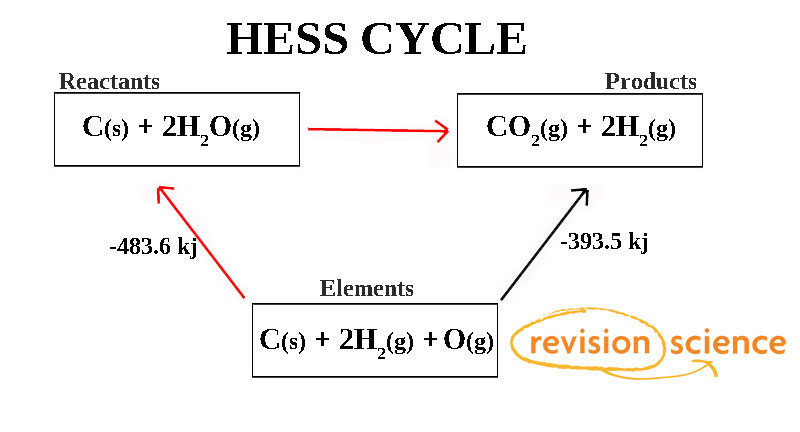 Hess S Law And Hess Cycles Chemistry A Level Revision
Hess S Law And Hess Cycles Chemistry A Level Revision





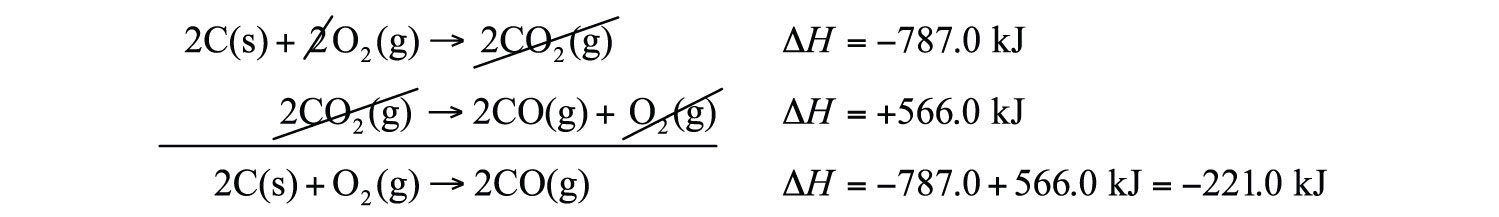



Posting Komentar
Posting Komentar