What Is The Trend For Electron Affinity
The trends noted here are very similar to those in ionization energy and change for similar though opposing reasons. Not all elements form stable negative ions in which case the electron affinity is zero or even positive.
Which Group Has The Highest Electron Affinity Quora
Electron affinity the energy associated with forming an anion is more favorable exothermic when electrons are placed into lower energy orbitals closer to the nucleus.
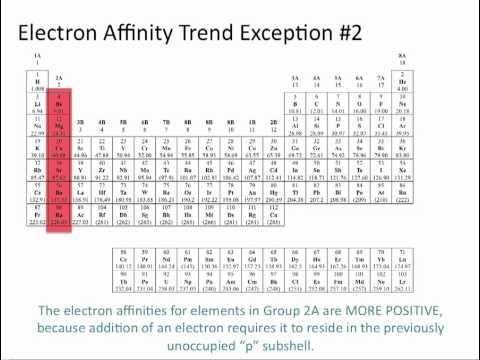
What is the trend for electron affinity. Electron affinities reported in unites of kilojoules per mole kj mol. The change is small and there are many exceptions. Electron affinity is the energy change that results from adding an electron to a gaseous atom.
Fluorine f has a higher electron affinity than oxygen o and so on. What is the general trend for electron affinity values going across a period. The trends for electron affinity are generalizations and so it s important to indicate a few exceptions.
It s important to mention that noble gases are not included in the trend for electron. Moving from left to right and bottom to top on the period table electron affinity increases. Therefore electron affinity becomes increasingly negative as we move left to right across the periodic table and decreases as we move down a group.
Electron affinity is the attraction a neutral atom has for a non bonding electron. The electron affinity of an element is the energy change which accompanies the addition of an electron to an atom in the gas phase to produce a negatively charged anion. Electron affinity becomes less negative down a group.
So the more negative the electron affinity the more favorable the electron addition process is. D the values show no trend. X g e x g.
The a values become more negative. The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. Electron affinity decreases or increases across a period depending on electronic configuration.
Because this value is negative energy is released we say that the electron affinity of fluorine is favorable. As the principal quantum number increases the size of the orbital increases and the affinity for the electron is less. Electron affinity follows the trend of electronegativity.
The electron affinity trend describes the trend across the periodic table and describes how much energy in an atom is released or spent when an electron is added to a neutral atom or the energy change that occurs when an electron is added to a neutral atom. As with ionization energy there are two rules that govern the periodic trends of electron affinities. B the values become more positive.
For example when a fluorine atom in the gaseous state gains an electron to form f g the associated energy change is 328 kj mol. This is because going from left to right and bottom to top the atomic radius decreases so it is easier for the nucleus to attract negative electrons. Data taken from john emsley the elements 3rd edition oxford.
C the values stay relatively constant. The electron affinity trend describes how as one follows the periodic table left to right electron affinity increases and how it usually decreases as one moves down a group of elements top to bottom.
Trends Electron Affinity Fundamentals Of Chemistry Ksu Studocu
Electron Affinity Definition Trends Equation Video Lesson
Webelements Periodic Table Periodicity Electron Affinity
History Of Chemistry Electron Affinity Sutori
Periodic Trends Electron Affinity Ck 12 Foundation
Periodic Trends Electron Affinity Youtube
Electron Affinity Introduction To Chemistry
Periodic Trends Electron Affinity Youtube
Introduction Of Electron Affinity Trend Definition Equation
7 5 Electron Affinities Chemistry Libretexts
Why Does Electron Affinity Decrease Across A Period How Can It Be
Electron Affinity Periodic Trends
Webelements Periodic Table Periodicity Electron Affinity
What Are The Periodic Trends For Atomic Radii Ionization Energy
Electron Affinity Trend Science Trends
Periodic Trends Electron Affinity Read Chemistry Ck 12
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcs3ahkkb5nasom 0yinhwbdbjemnluyf Kywdcahjeh2z6cw4ex Usqp Cau
Electron Affinity Periodic Trends
Electron Affinity Chemistry Libretexts
Why Do Halogens Have A High Electron Affinity Quora
Periodic Behavior Presentation Chemistry
Periodic Trends Electron Affinity Chemistry Video Clutch Prep
Periodic Trends Electron Affinity
Electron Affinity Chemistry Tutorial Youtube
Electron Affinity Google Search Electron Affinity Electrons
Ionization Energy And Electron Affinity
Electron Affinity Trend Science Trends
Trends In The Periodic Table Course Hero
Electron Affinity Periodic Table Definition Trends
What Are The Observed Periodic Trends In E Clutch Prep
Why Does The Electron Affinity Increase Become More Exothermic
Electron Affinity Trends Of The Periodic Table
Electron Affinity Chemistry Libretexts
Posting Komentar
Posting Komentar