Koh Acid Or Base Or Neutral
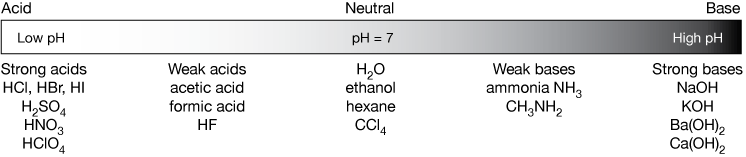
Koh h2so3 k2so3 h2o it is also useful to have memorized the common. For something like hcl an acid the ph would be below 7.
 Ammonium Fluoride General Chemistry Solved Quiz Docsity
Ammonium Fluoride General Chemistry Solved Quiz Docsity
First we need to figure out the acid and base that were neutralized to form potassium sulfite.
Koh acid or base or neutral. You have already determined that k is the very weak conjugate acid of koh and will not affect the ph of a solution. Identify each salt as acidic basic or neutral. In chemistry a salt is an ionic compound that can be formed by the neutralization reaction of an acid and a base.
Salts of. Therefore neither ion will affect the acidity of the solution so kcl is a neutral salt. The equation for k2so3 is.
Nh3 nh4oh note that we are talking about whether nh4no3 is an acid base or neutral when dissolved in water. Complete the following statements. If we tested the ph it would be well above 7 meaning that koh is a base.
Hco 2 h koh khco 2 h 2 o. Nh3 nh4oh note that we are talking about whether kno3 is an acid base or neutral when dissolved in water. When formic acid reacts with potassium hydroxide sodium formate is formed.
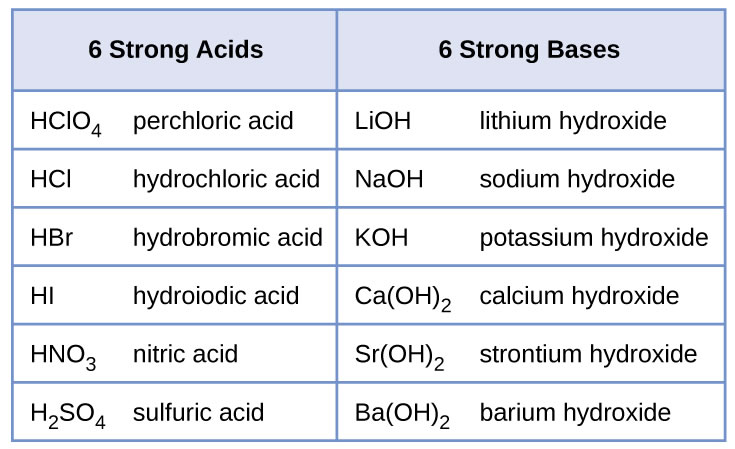
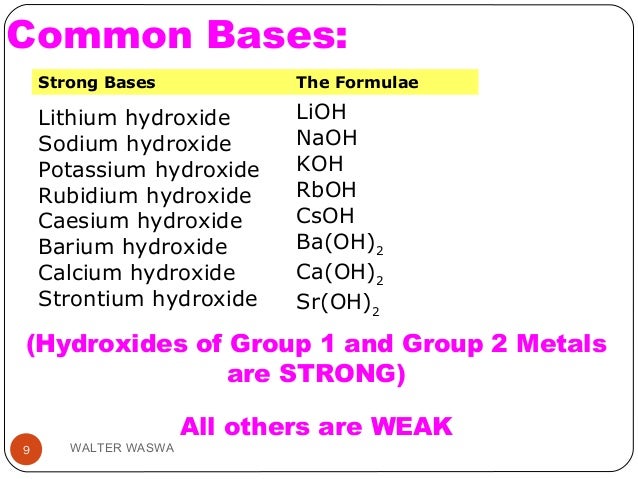
Lioh naoh koh ca oh 2 sr oh 2 ba oh 2 weak bases. Although the k ion derives from a strong base koh the no 2 ion derives from a weak acid hno 2. Salts of strong.
Koh is also called caustic potash or potash lye and can be made by. Therefore neither ion will affect the acidityof the solution so kclis a neutralsalt. The ions from kclderive from a strong acid hcl and a strong base koh.
Salts are composed of related numbers of cations positively charged ions and anions negative ions so that the product is electrically neutral without a net charge. Lioh naoh koh ca oh 2 sr oh 2 ba oh 2 weak bases. The ions from kcl derive from a strong acid hcl and a strong base koh.
Therefore the solution will be basic and kno 2 is a basic salt. Salts of strong bases and weak acids. Therefore neither ion will affect the acidity of the solution so kcl is a neutral salt.
Although the k ion derives from a strong base koh the no 2 ion derives from a weak acid hno 2. Although the k ion derives from a strong base koh the no 2 ion derives from a weak acid hno 2. What about hco 2.
The ions from kcl derive from a strong acid hcl and a strong base koh.
 Is Koh An Acid Or Base Youtube
Is Koh An Acid Or Base Youtube
Hydrolysis Of Acid And Base Pages 1 4 Text Version Anyflip
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gct84xlgyet 0xutwbhyaosmk Nyrdhhleghttbetxdnzathldh0 Usqp Cau
 Answered Classify The Following As Either Bartleby
Answered Classify The Following As Either Bartleby
 Types Of Reactions Precipitates And Acid Base
Types Of Reactions Precipitates And Acid Base
 Acids Bases Salts Acid Base Equilibrium Ppt Video Online
Acids Bases Salts Acid Base Equilibrium Ppt Video Online
 Use The Information In The Tables To Determine Whether The
Use The Information In The Tables To Determine Whether The
 Chemistry 100 Chapter 14 Acids And Bases Ppt Download
Chemistry 100 Chapter 14 Acids And Bases Ppt Download
 Is Koh An Acid Or Base Or Neutral
Is Koh An Acid Or Base Or Neutral

Is Potassium Carbonate An Acidic Salt Quora
13 3 Finding The Ph Of Weak Acids Bases And Salts Chemistry
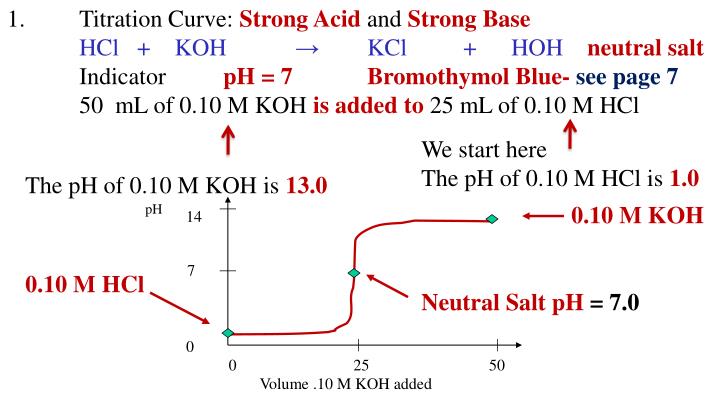 Ppt Acids Lesson 18 Titration Curves Powerpoint Presentation
Ppt Acids Lesson 18 Titration Curves Powerpoint Presentation
 Is Koh An Acid Or Base Youtube
Is Koh An Acid Or Base Youtube
 Is Ki Acidic Basic Or Neutral Dissolved In Water Youtube
Is Ki Acidic Basic Or Neutral Dissolved In Water Youtube
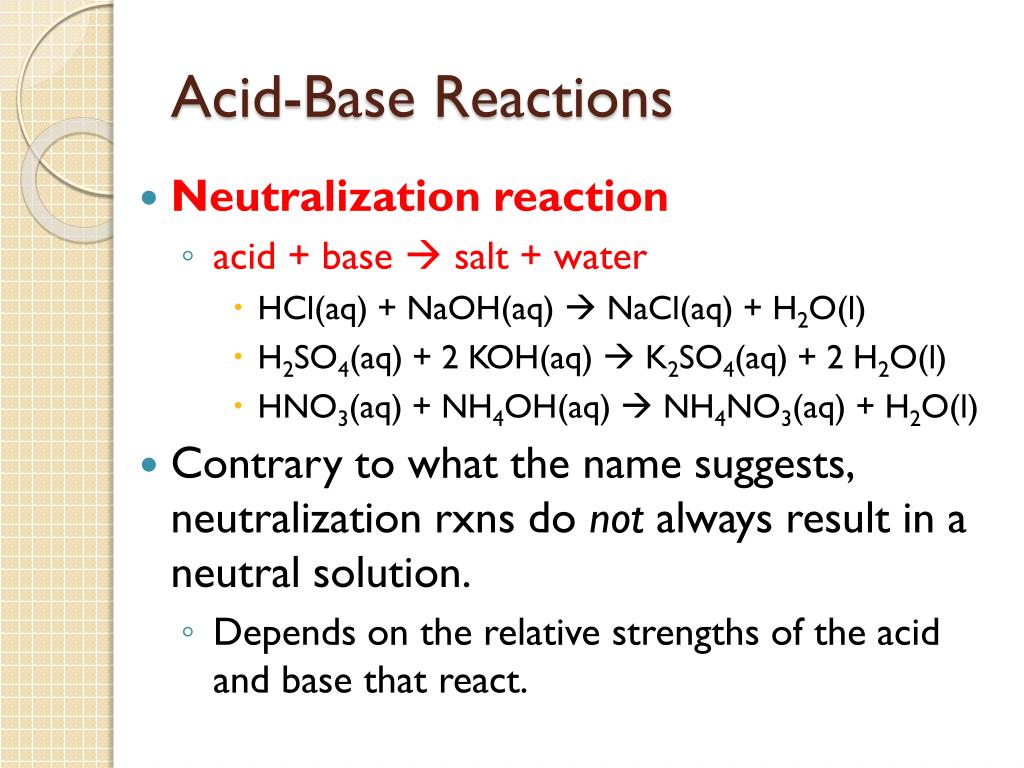 Ppt Acid Base Reactions Powerpoint Presentation Free Download
Ppt Acid Base Reactions Powerpoint Presentation Free Download
 Modern Theories Of Acids Bases The Arrhenius And Bronsted Lowry
Modern Theories Of Acids Bases The Arrhenius And Bronsted Lowry
 Packet 10 Acids Bases And Salts Reference Tables K L M J
Packet 10 Acids Bases And Salts Reference Tables K L M J
 Acids And Bases I Introduction
Acids And Bases I Introduction
 8 6 Acid Base Properties Of Salt Solutions
8 6 Acid Base Properties Of Salt Solutions
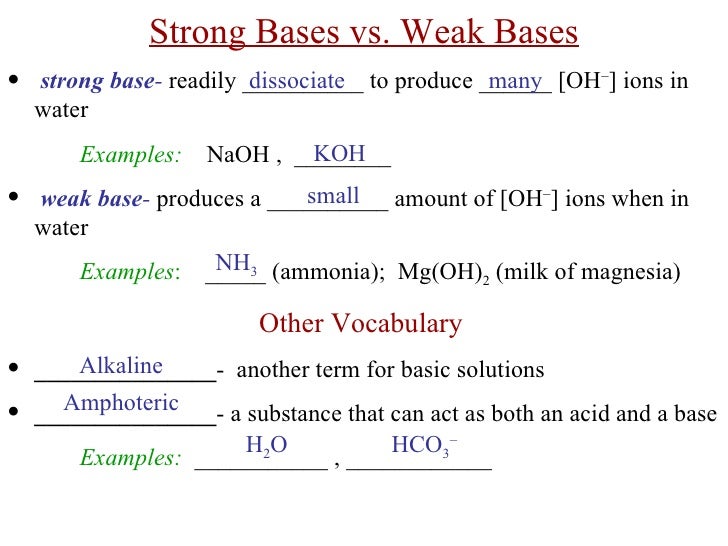 Ch 20 21 Notes Acids Bases Teacher
Ch 20 21 Notes Acids Bases Teacher
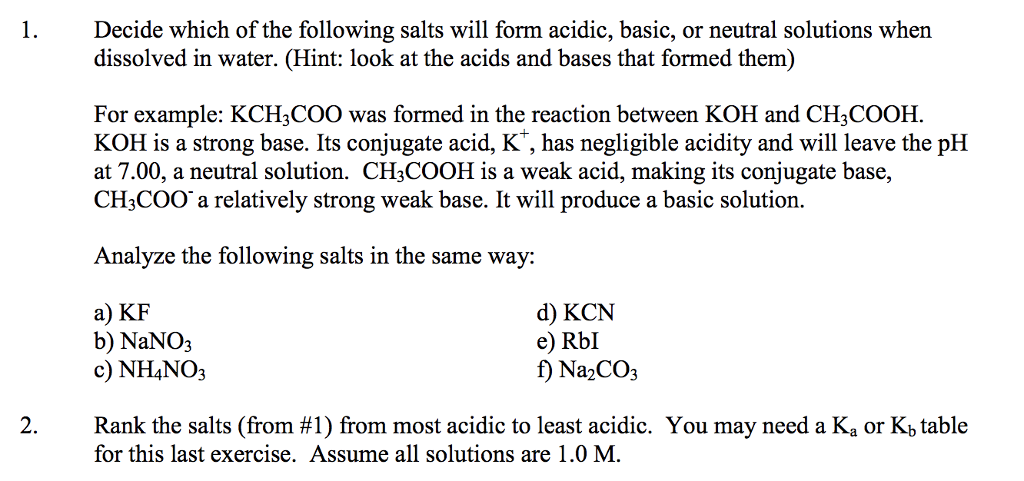 Solved 1 Decide Which Of The Following Salts Will Form Ac
Solved 1 Decide Which Of The Following Salts Will Form Ac
 Video Applying Knowledge Of The Chemical Behavior Of Kbr Nagwa
Video Applying Knowledge Of The Chemical Behavior Of Kbr Nagwa
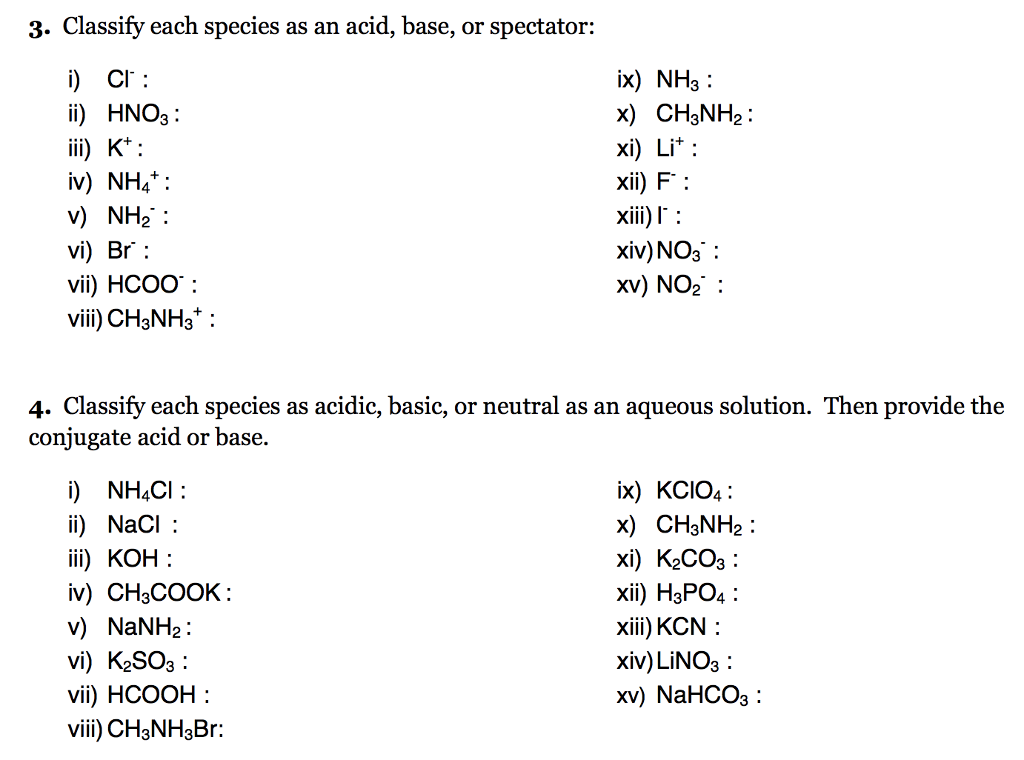 Solved 3 Classify Each Species As An Acid Base Or Spec
Solved 3 Classify Each Species As An Acid Base Or Spec
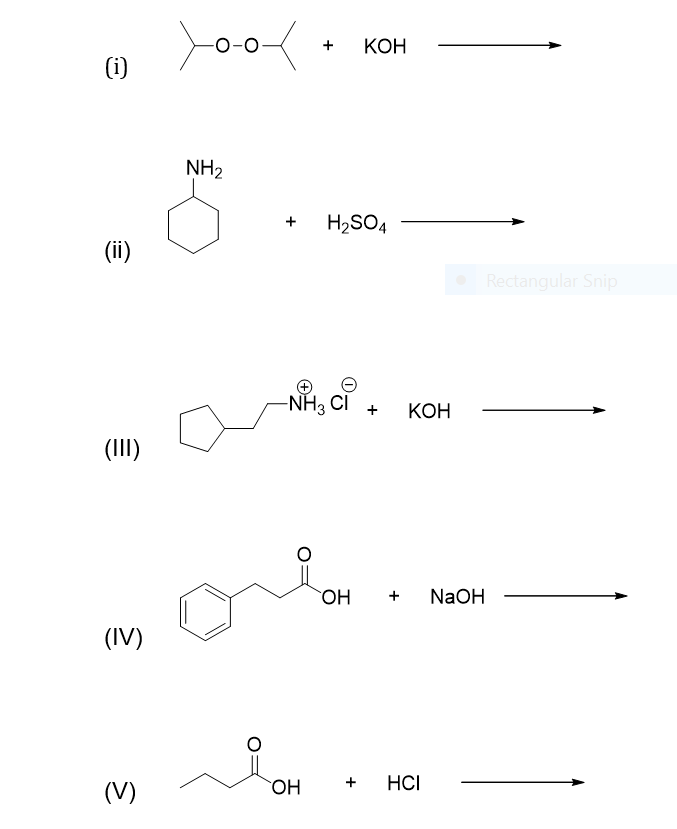 Solved Label Each Of The Compounds As Acid Base Conjuga
Solved Label Each Of The Compounds As Acid Base Conjuga
 Given Salt Find Acid And Base Video Khan Academy
Given Salt Find Acid And Base Video Khan Academy
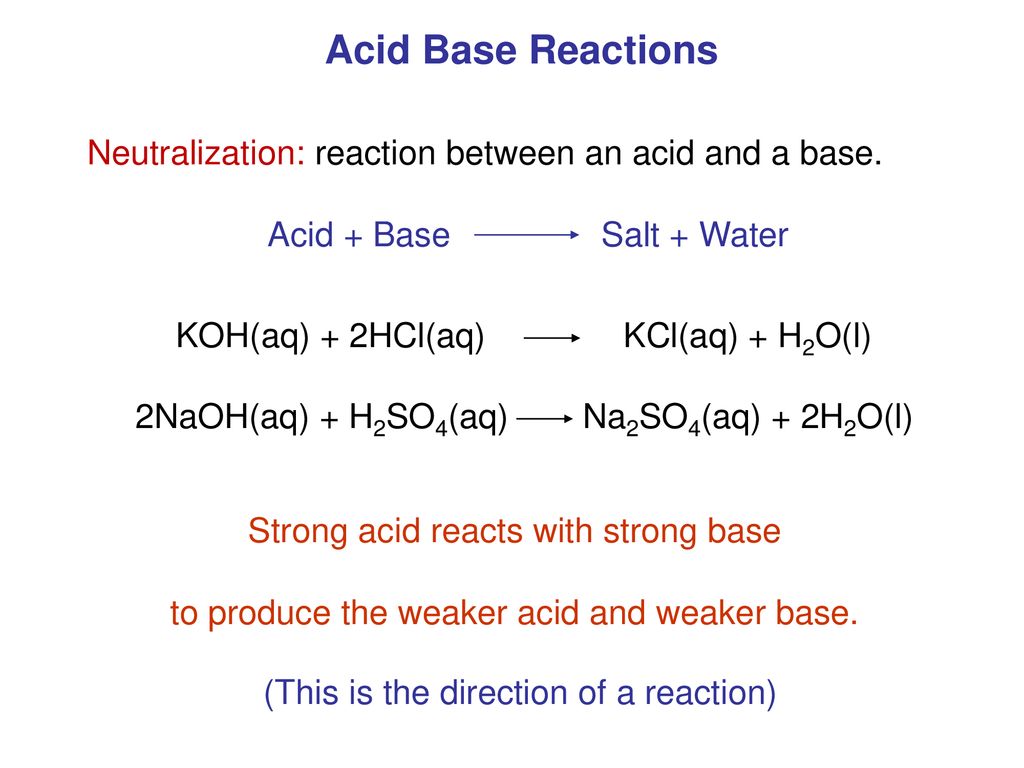
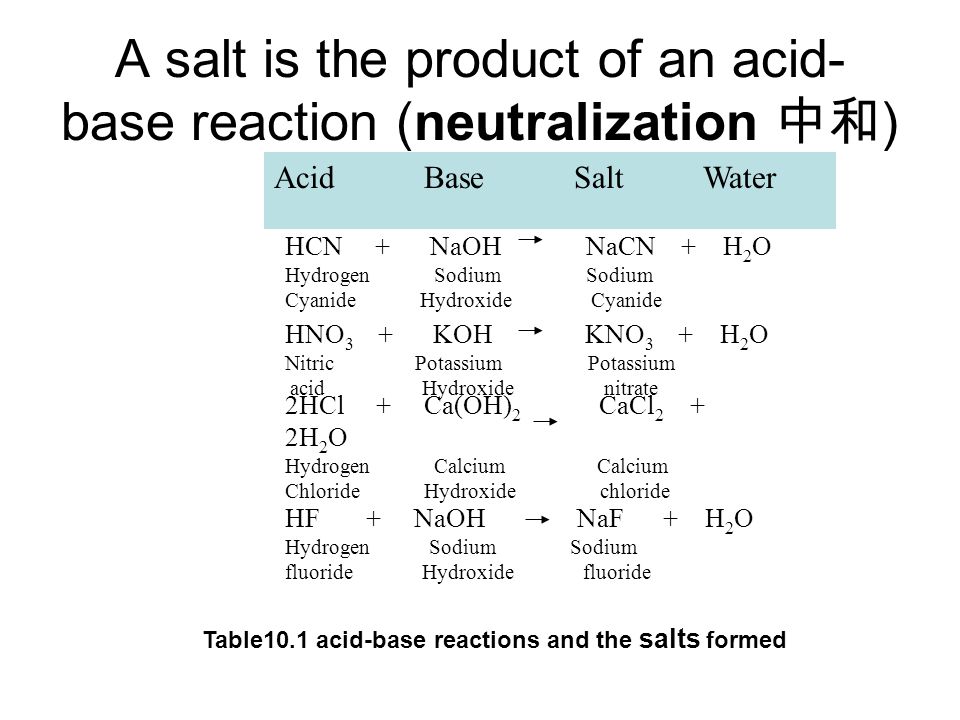
 Neutralization Reaction Definition Equation Examples Video
Neutralization Reaction Definition Equation Examples Video
 Please Help To Find The Ph A Chemist Titrates 2200 Ml Of A 05950 M
Please Help To Find The Ph A Chemist Titrates 2200 Ml Of A 05950 M
 Answered Hases And Compound X Will Be Found Bartleby
Answered Hases And Compound X Will Be Found Bartleby
Acid Base Chemistry Chemistry Encyclopedia Reaction Water

Acid Base Salt Difference Quora



Posting Komentar
Posting Komentar