First Order Reaction Graph
For first order reaction we know that. Differential rate laws are generally used to describe what is occurring on a molecular.
First write the differential form of the rate law.
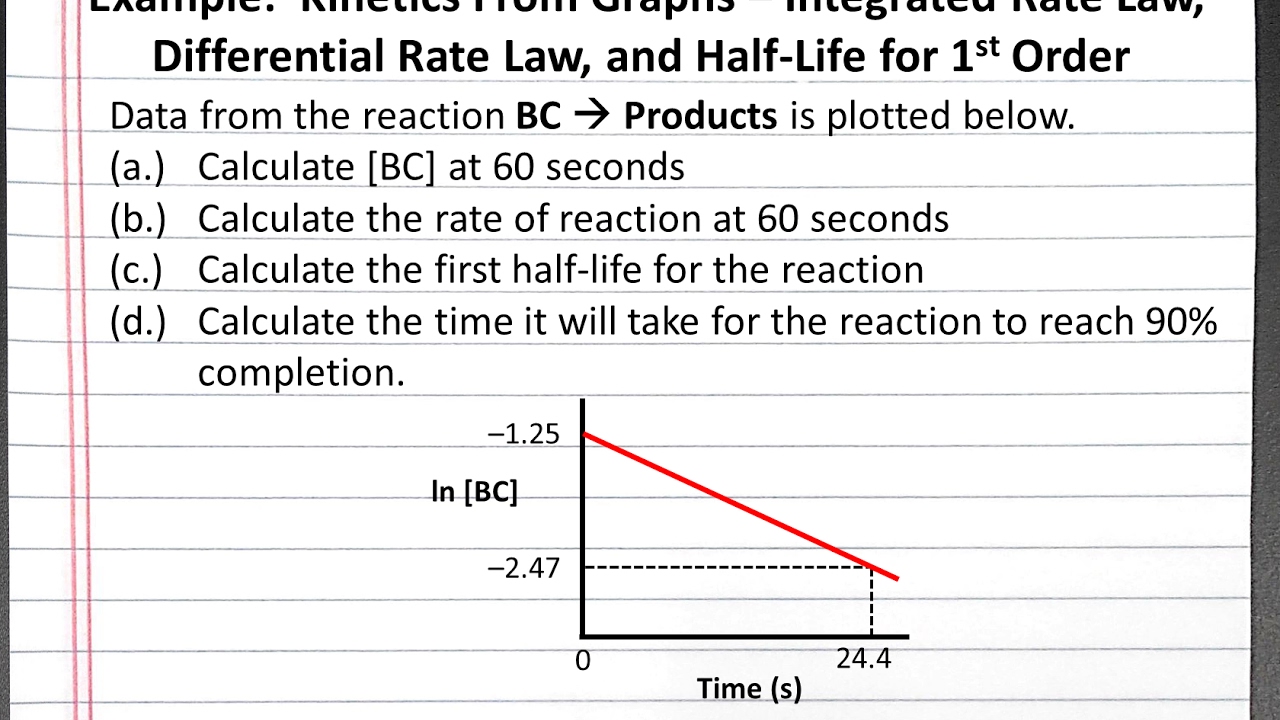
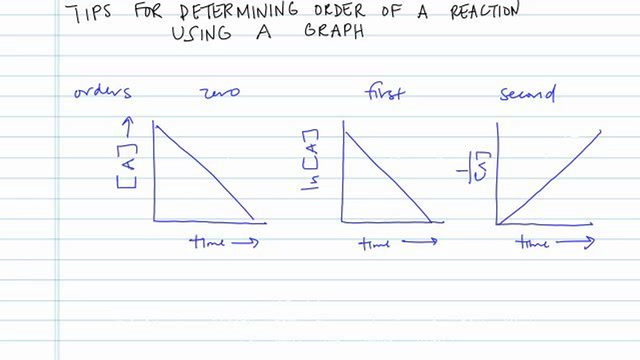
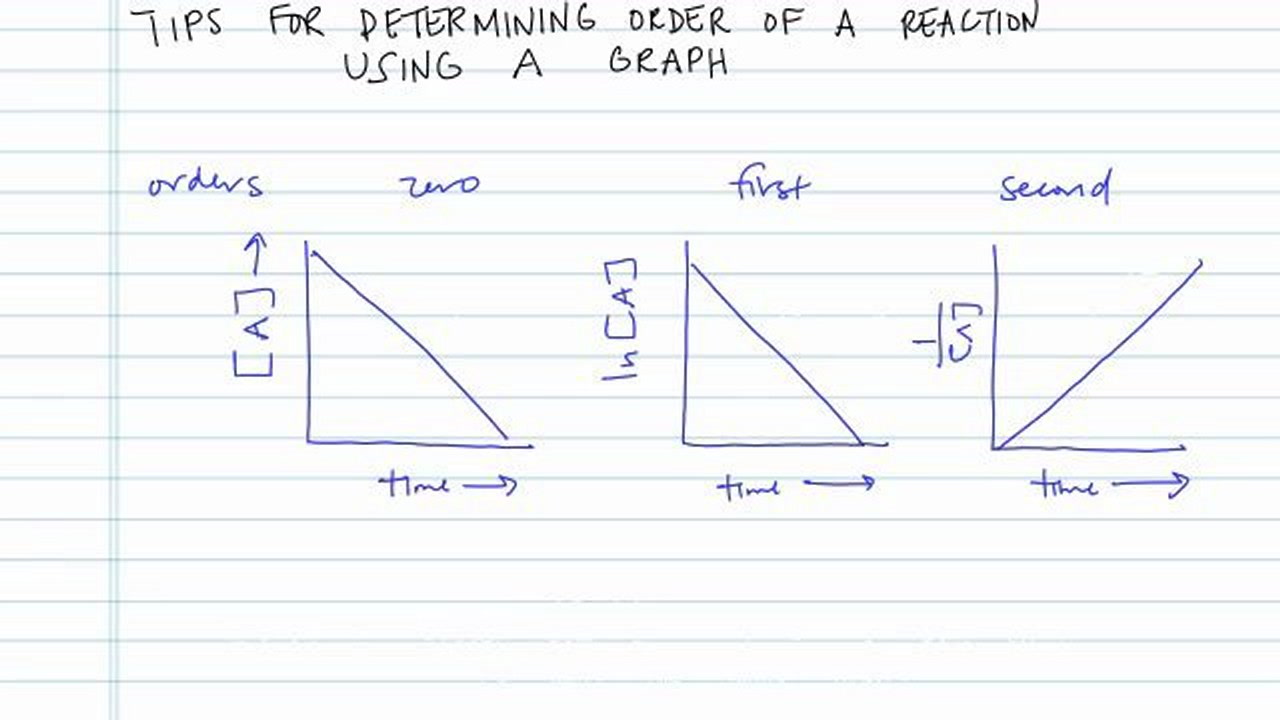
First order reaction graph. Compare the graphs with those in figure 14 16 properties of reactions that obey zeroth first and second order rate laws to determine the reaction order. B write the rate law for the reaction. So in a zero order if i graphed my data then on my y axis i ll have the concentration of whatever my reactant is.
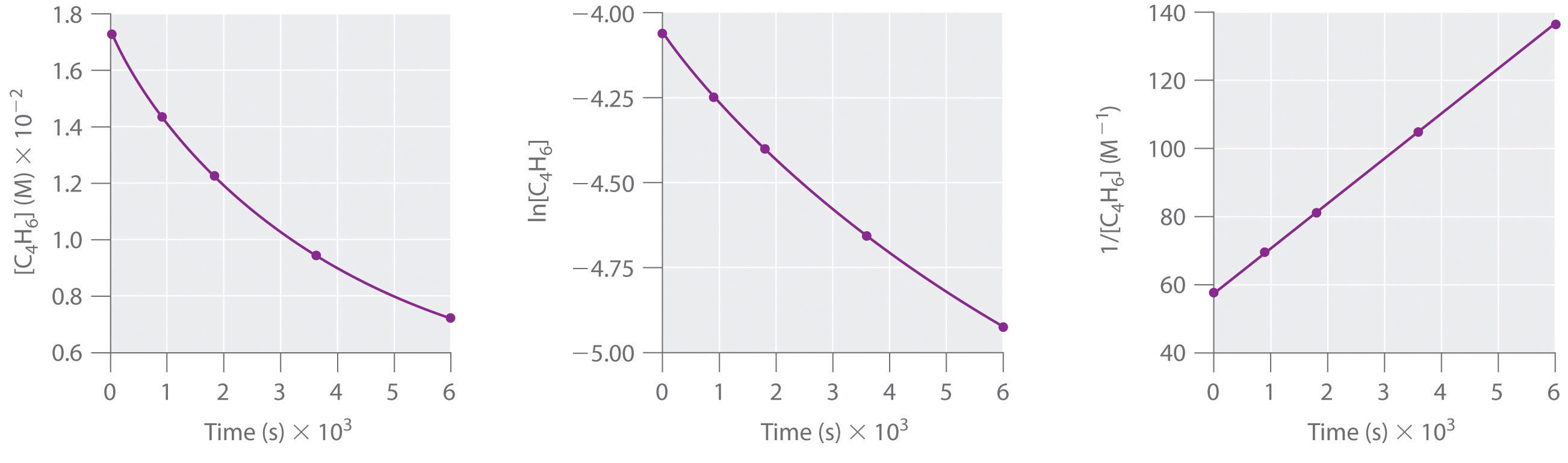
Voiceover let s see how to plot data for a first order reaction so the conversion of cyclopropane into propene is a first order reaction and in part a they want us to use the experimental data to show that it s first order so we look at the data over here and we can see as time increases right the concentration of cyclopropane decreases which makes sense because cyclopropane is. So for your graphs the orders of the reaction that we re going to take a look at are. This means if we start with 4 mole l 1 of a reactant reacting by first order.
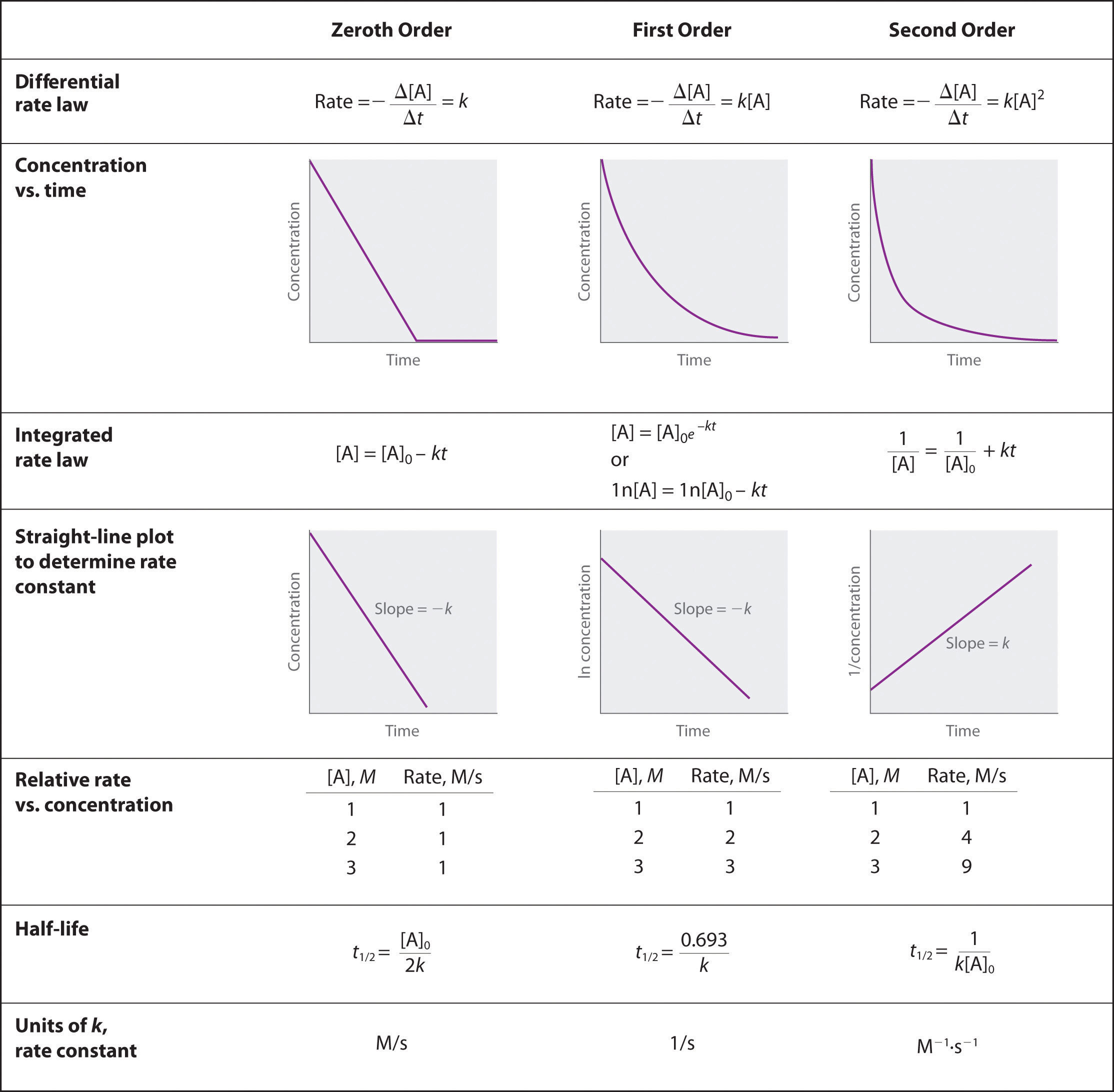
At half life of reaction t t a a o 2. Analyzing the rate equation. For a second order reaction a plot of the inverse of the concentration of a reactant versus time is a straight line with a slope of k.
In fractional order reactions the order is a non integer which often indicates a chemical chain reaction or other complex reaction mechanism. Where m stands for concentration in molarity mol l 1 t for time and k for the reaction rate constant. Uok unit 5 ru unit 5 welcome to scientia institute kota.
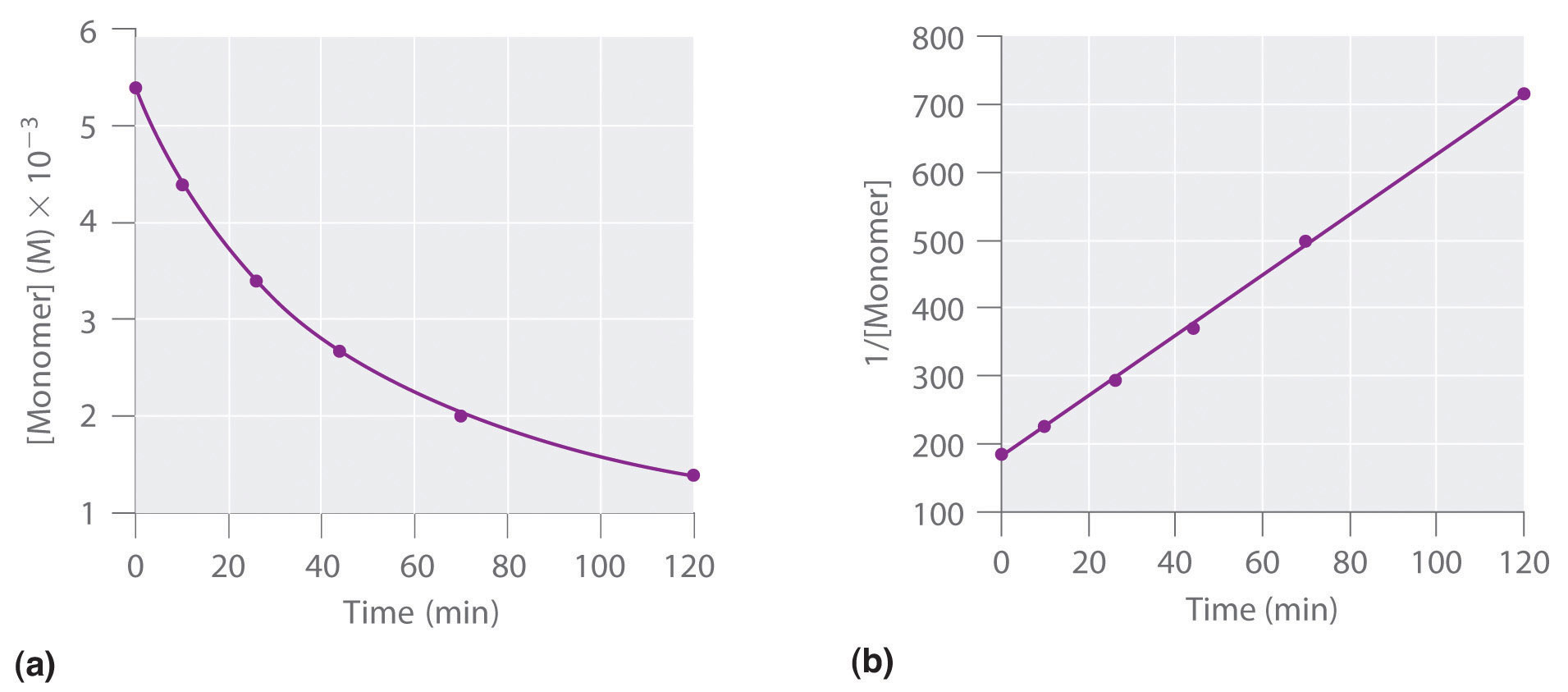
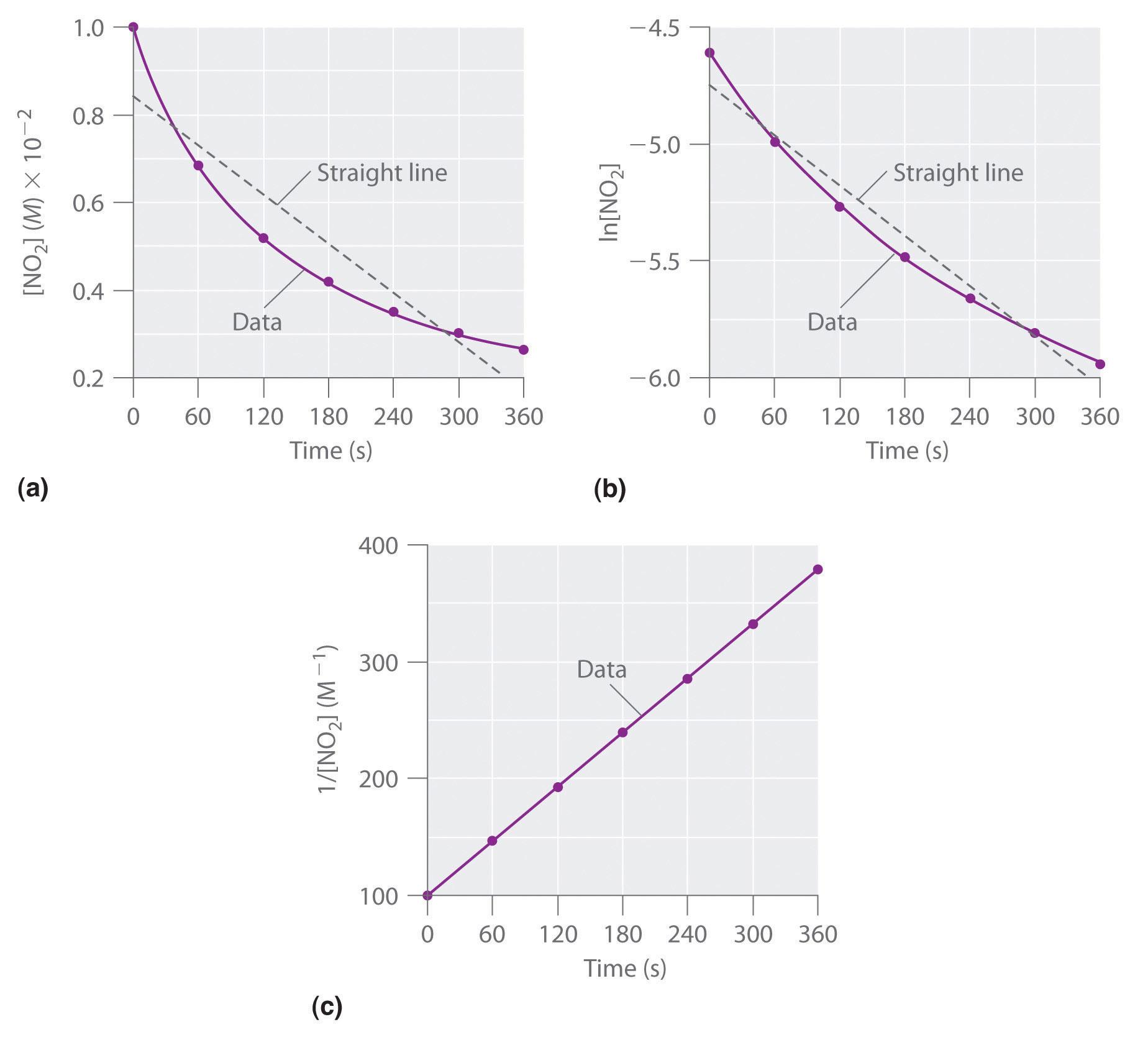
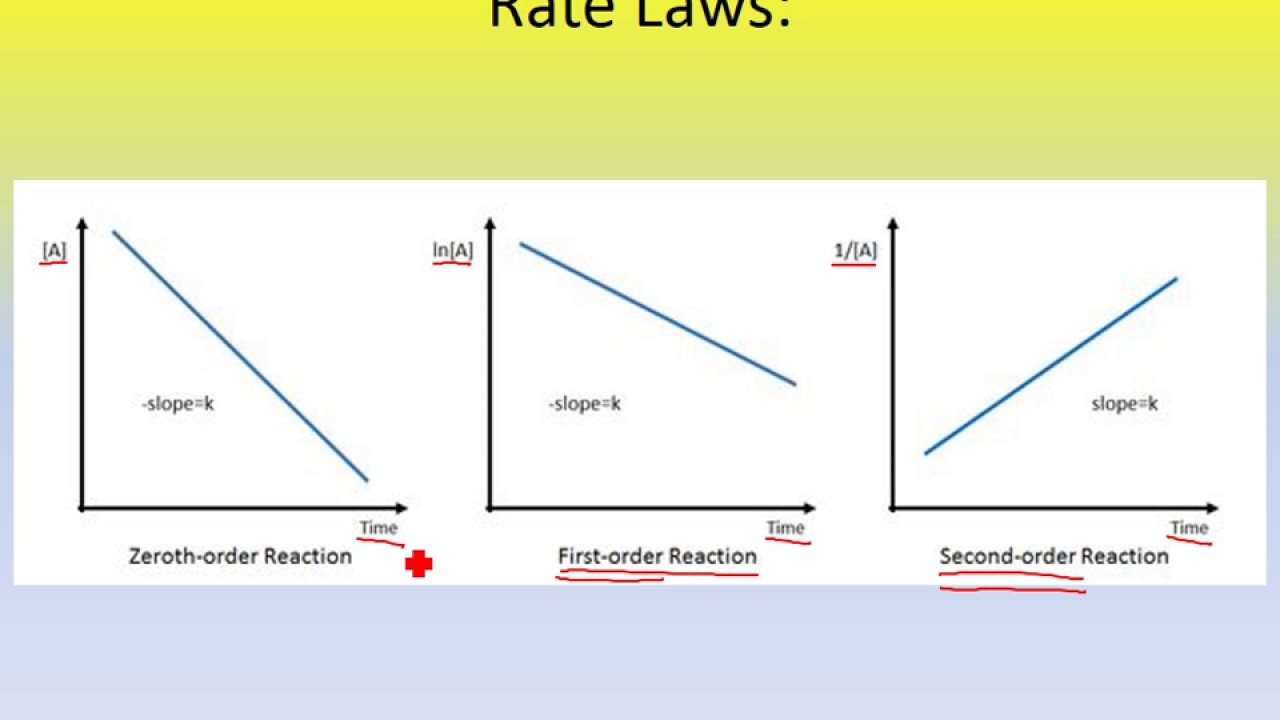
Using the appropriate data from the table and the linear graph corresponding to the rate law for the reaction calculate the slope of the plotted. That ll be graphed versus time that i have there. For a first order reaction a plot of the natural logarithm of the concentration of a reactant versus time is a straight line with a slope of k.
We are providing best study material for bsc which is useful for both university of kota and university of rajasthan students. For a zero order reaction rate k. Identify the rate equation from the reaction.
Then you can choose the correct rate equation. Second integrate both sides of the. First order reactions the differential representation.
Find the variables that create a linear graph of the reaction. Since k is a constant for a given reaction at a given temperature and the expression lacks any concentration term so half time of a 1st order reaction is a constant independent of initial concentration of reactant. So based on your concentrational increase here.
The graph that is linear indicates the order of thereaction with respect to a. The half life of a first order reaction is often expressed as t 1 2 0 693 k as ln 2 0 693. K slope of line for a 1storder reaction rate k a k slope of line for a 2ndorder reaction.
The rate equation can help you. How to determine order of reaction method 1 of 3. Zero order first order and second order.
 Methods Of Determining Reaction Order
Methods Of Determining Reaction Order
 Using Graphs To Determine Rate Laws Rate Constants And Reaction
Using Graphs To Determine Rate Laws Rate Constants And Reaction
 Chemical Kinetics 9 Graph For First Order Reaction Youtube
Chemical Kinetics 9 Graph For First Order Reaction Youtube
 Determining Order Of A Reaction Using A Graph Concept
Determining Order Of A Reaction Using A Graph Concept
 Determining Order Of A Reaction Using A Graph Concept
Determining Order Of A Reaction Using A Graph Concept
 Using Graphs To Determine Integrated Rate Laws Chemistry Libretexts
Using Graphs To Determine Integrated Rate Laws Chemistry Libretexts
Concentration Time Relationships Integrated Rate Laws
17 2 Reaction Rates Typically Change With Time Chemistry Libretexts
 Pseudo First Order Reaction Rate Law Order Of Reaction Examples
Pseudo First Order Reaction Rate Law Order Of Reaction Examples
 Determining The Order Of The Reaction From Graphs Adv Chem Ch 5
Determining The Order Of The Reaction From Graphs Adv Chem Ch 5
 Chemistry 201 Kinetics From Graphs For First Order Reaction Youtube
Chemistry 201 Kinetics From Graphs For First Order Reaction Youtube
 Using Graphs To Determine Rate Laws Rate Constants And Reaction
Using Graphs To Determine Rate Laws Rate Constants And Reaction
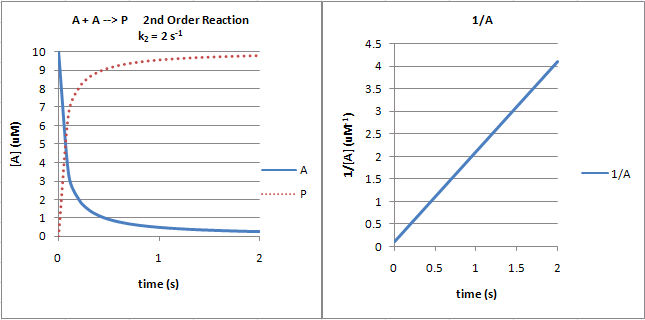 B1 Single Step Reactions Biology Libretexts
B1 Single Step Reactions Biology Libretexts
 Using Graphs To Determine Rate Laws Rate Constants Reaction
Using Graphs To Determine Rate Laws Rate Constants Reaction
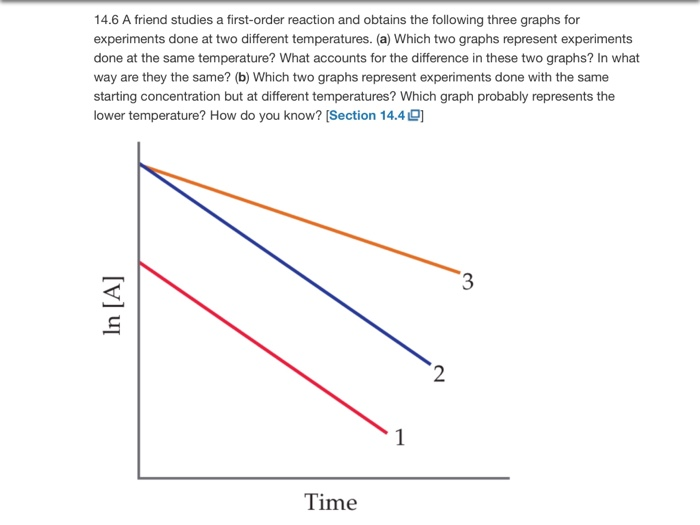 Solved 14 6 A Friend Studies A First Order Reaction And O
Solved 14 6 A Friend Studies A First Order Reaction And O
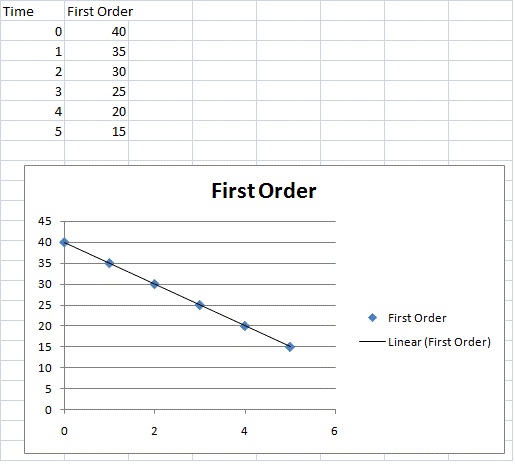 Chm1 13 The Integrated Rate Equations Collection
Chm1 13 The Integrated Rate Equations Collection
 Solved 3 Kinetic Data For A Particular First Order React
Solved 3 Kinetic Data For A Particular First Order React
 Pseudo First Order Reaction Order Of Reaction Examples With Videos
Pseudo First Order Reaction Order Of Reaction Examples With Videos
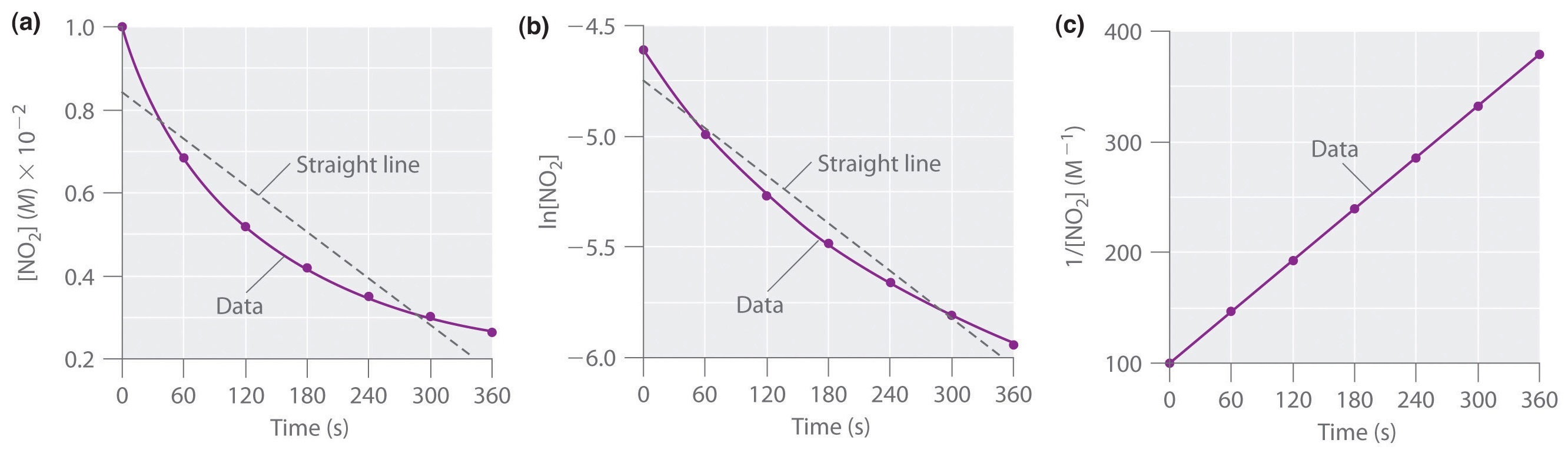 Using Graphs To Determine Integrated Rate Laws Chemistry Libretexts
Using Graphs To Determine Integrated Rate Laws Chemistry Libretexts
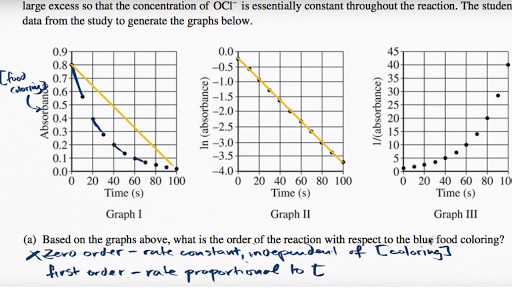
 Savvy Chemist Reaction Kinetics 5 Kinetics And Mechanism
Savvy Chemist Reaction Kinetics 5 Kinetics And Mechanism
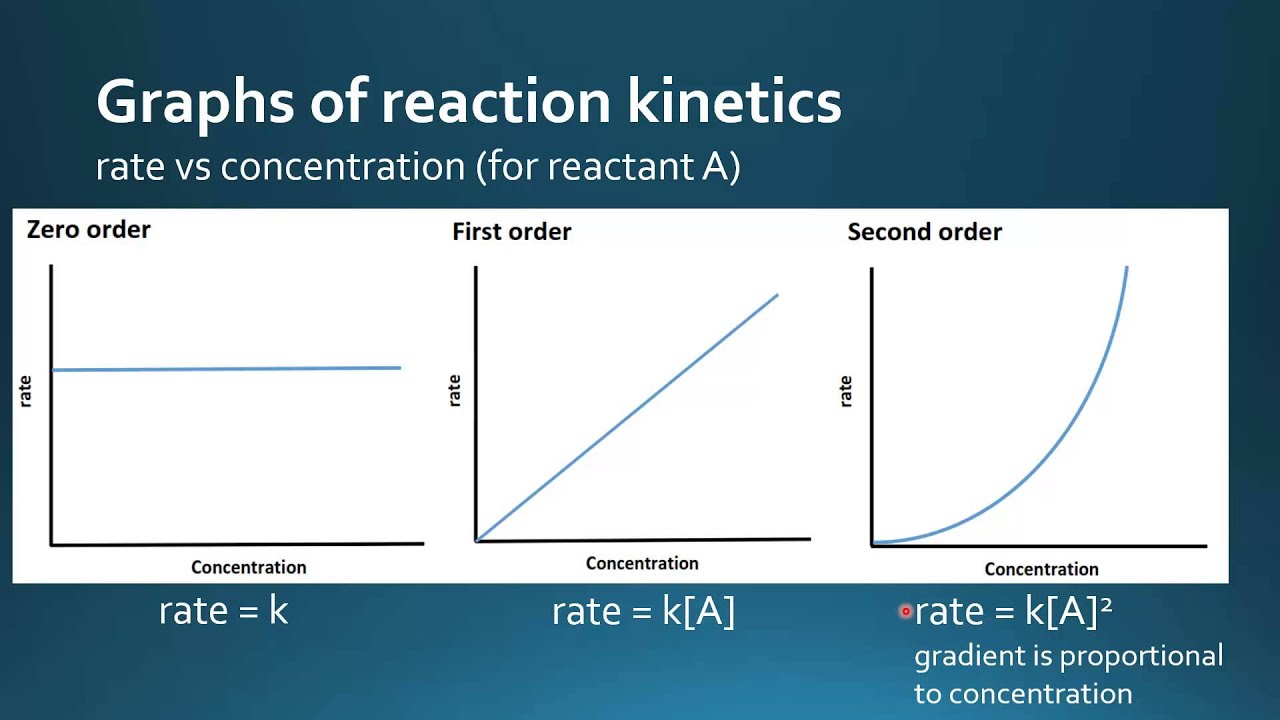 16 1 4 Sketch Graphical Representations For Zero First And
16 1 4 Sketch Graphical Representations For Zero First And
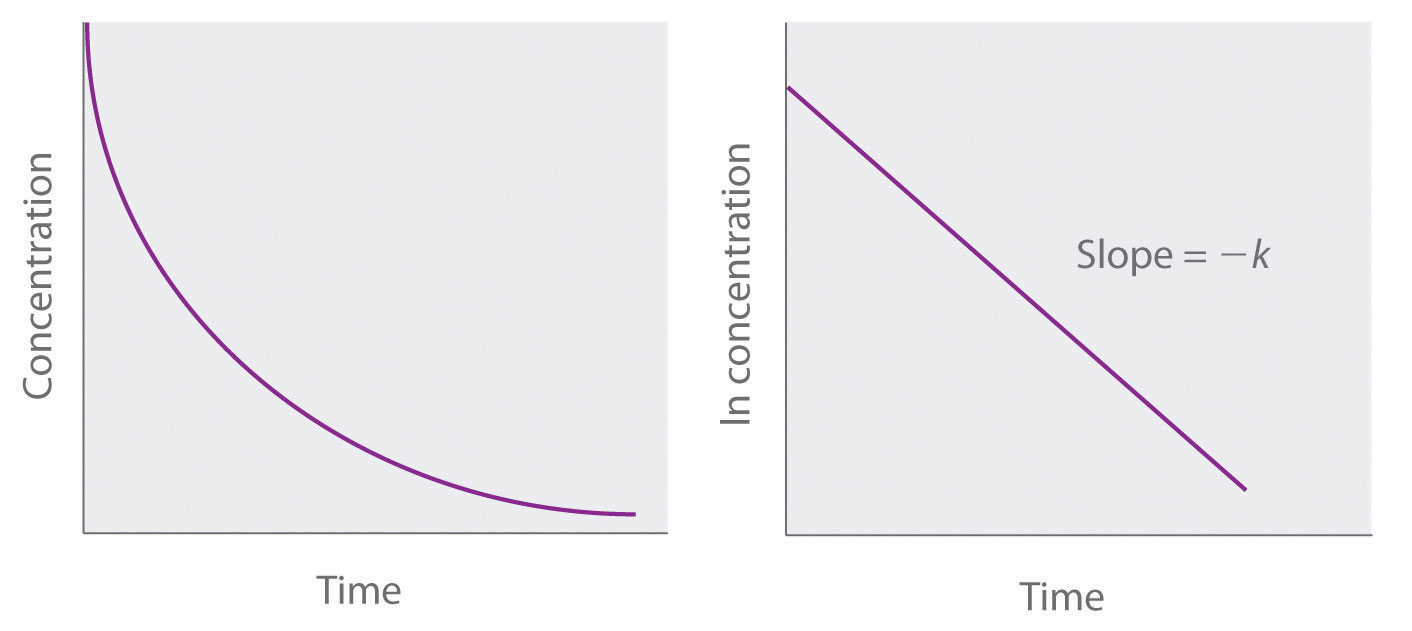 Methods Of Determining Reaction Order
Methods Of Determining Reaction Order
Concentration Time Relationships Integrated Rate Laws
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctpyez7wqfrtp23wmjugwt E0irkcv2mjbqrhkkz2ux1tfbdrwc Usqp Cau
 Determination Of The Pseudo First Order Reaction Constant K App
Determination Of The Pseudo First Order Reaction Constant K App
 Pseudo First Order Of The Reaction Rate In Photocatalytic State
Pseudo First Order Of The Reaction Rate In Photocatalytic State
 Why Is The First Order Reaction Linear Quora
Why Is The First Order Reaction Linear Quora
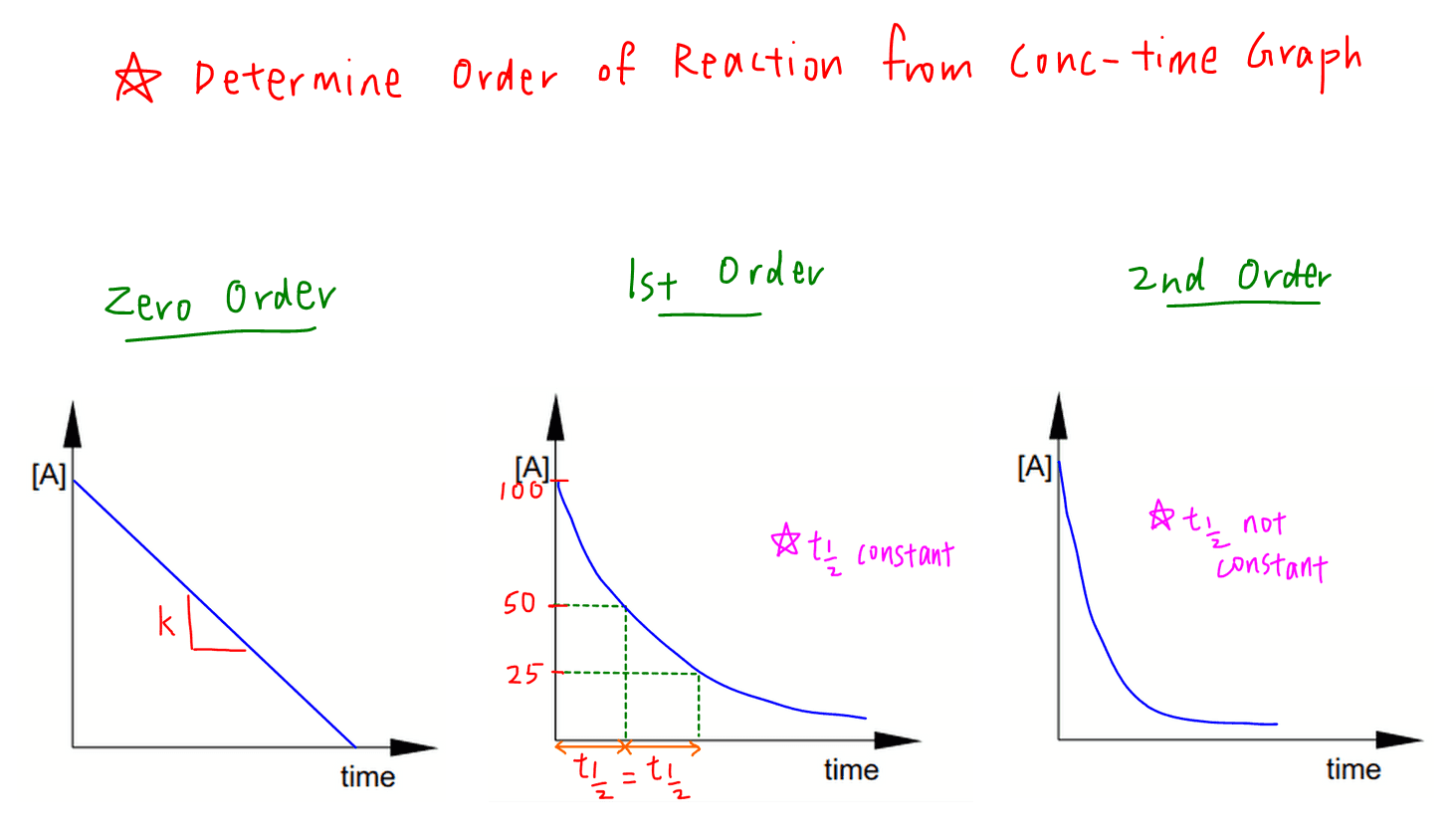 Rate Equation And Order Of Reaction
Rate Equation And Order Of Reaction
 Is The Cooling Of Water A First Order Process Chemhelp
Is The Cooling Of Water A First Order Process Chemhelp
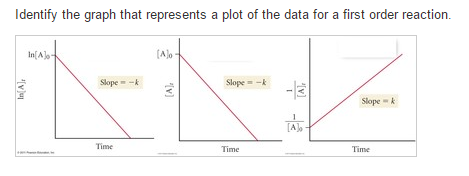 Solved Identify The Graph That Represents A Plot Of The D
Solved Identify The Graph That Represents A Plot Of The D
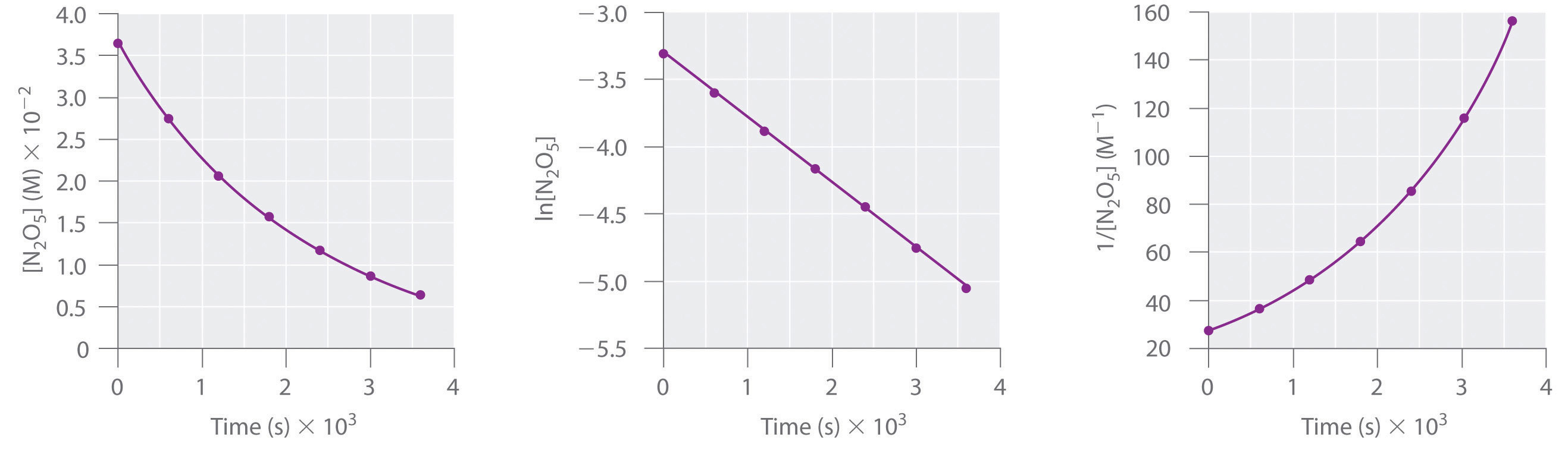 Using Graphs To Determine Integrated Rate Laws Chemistry Libretexts
Using Graphs To Determine Integrated Rate Laws Chemistry Libretexts
 Second Order Reaction Definition And Derivation For Rate Law And
Second Order Reaction Definition And Derivation For Rate Law And
Https Www Ohsd Net Cms Lib Wa01919452 Centricity Domain 594 2 208 20notes Pdf

Posting Komentar
Posting Komentar