Empirical To Molecular Formula
One molecule of glucosecontains 6 atoms of carbon 12 atoms of hydrogen and 6 atoms of oxygen. A empirical formula b molecular formula empirical formula of a compound gives the simplest whole number ratio of atoms of each element present in the compound.
 Difference Between Empirical And Molecular Formula Teaching
Difference Between Empirical And Molecular Formula Teaching
Use the molar massyou get by.

Empirical to molecular formula. It isn t the same as the molecular formula which tells you the actual number of atoms of each element present in a molecule of the compound. To calculate the empirical formula enter the composition e g. Percentages can be entered as decimals or percentages i e.
The molecular formula gives the actual whole number ratio between elements in a compound. Empirical formulae and molecular formulae. For the purposes of determining empirical formulas assume that we have 100 grams of the compound.
Empirical and molecular formula key takeaways the empirical formula gives the smallest whole number ratio between elements in a compound. 50 can be entered as 50 or 50 to determine the molecular formula enter the appropriate value for the molar mass. A chemical analysis of a sample of methyl acetate provides the following elemental data.
Examples of molecular and empirical formulas the molecular formulaof glucose is c6h12o6. The empirical formula for a chemical compound is an expression of the relative abundances of the elements that form it. For some molecules the empirical and molecular formulas are the same.
Enter an optional molar mass to find the molecular formula. For example a molecule with the empirical formula ch 2 o has an empirical formula mass of about 30 g mol 12 for the carbon 2 for the two hydrogens 16 for the oxygen. Molecular formulas are associated with gram molecular masses that are simple whole number multiples of the corresponding empirical formula mass.
The key difference between empirical and molecular formulas is that an empirical formula only gives the simplest ratio of atoms whereas a molecular formula gives the exact number of each atom in a molecule. 28 03 mg 21 60 si 1 16 h and 49 21 o. A compound can be represented by two types of chemical formulae.
How to determine empirical formula begin with the number of grams of each element which you usually find in an experiment or have given in a problem. You can derive the molecular formula of a compound from its empirical formula only if you know the molar mass of the compound. The molar mass for chrysotile is 520 8 g mol.
Determine the empirical and molecular formula for chrysotile asbestos. Different compounds with very different properties may have the same empirical formula. Hexane s molecular formula is c 6 h 14 and its empirical formula is c 3 h 7 showing a c h ratio of 3 7.
In chemistry we often use symbols to identify elements and molecules. Chrysotile has the following percent composition. C 40 h 6 67 o 53 3 of the compound.
To make the calculation easier assume the total mass of a sample is 100 grams so you can work with simple percentages. 48 64 carbon c 8 16 hydrogen h and 43 20 oxygen o. If this is the case the percentages will be equal to the mass of each element in grams.
Molecular formula of a.
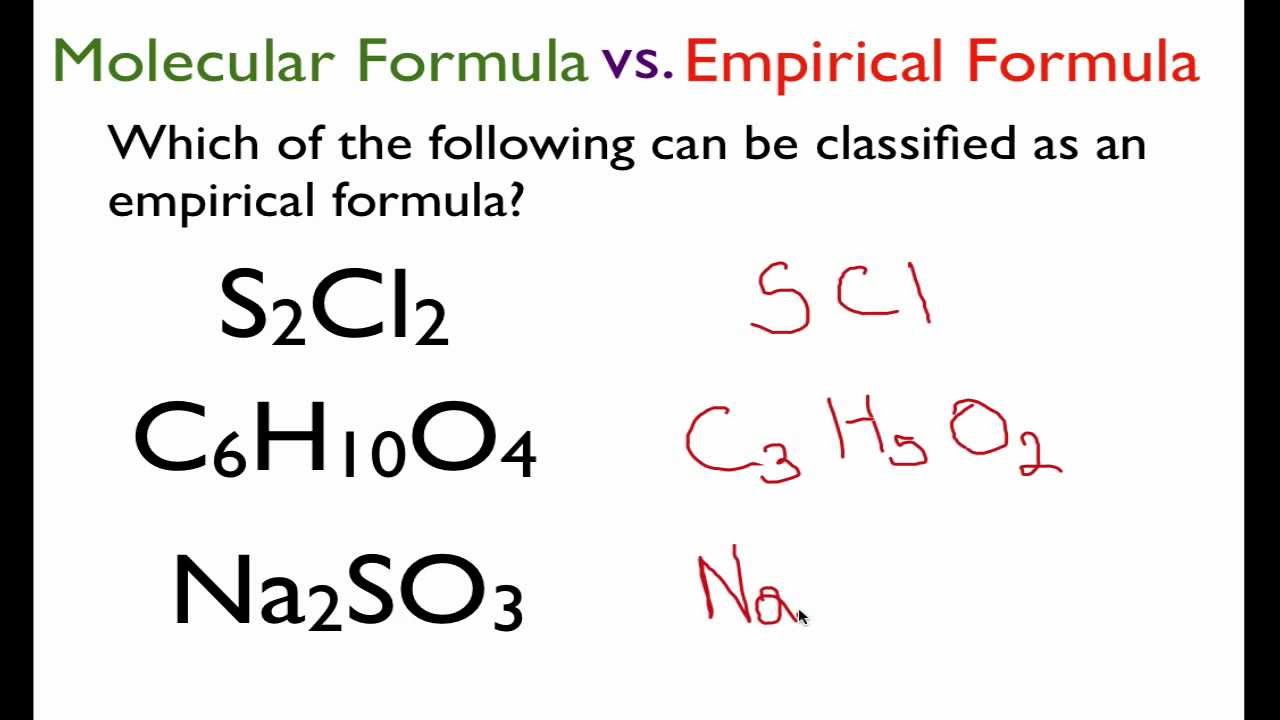 From The Molecular Formula To The Empirical Formula Youtube
From The Molecular Formula To The Empirical Formula Youtube
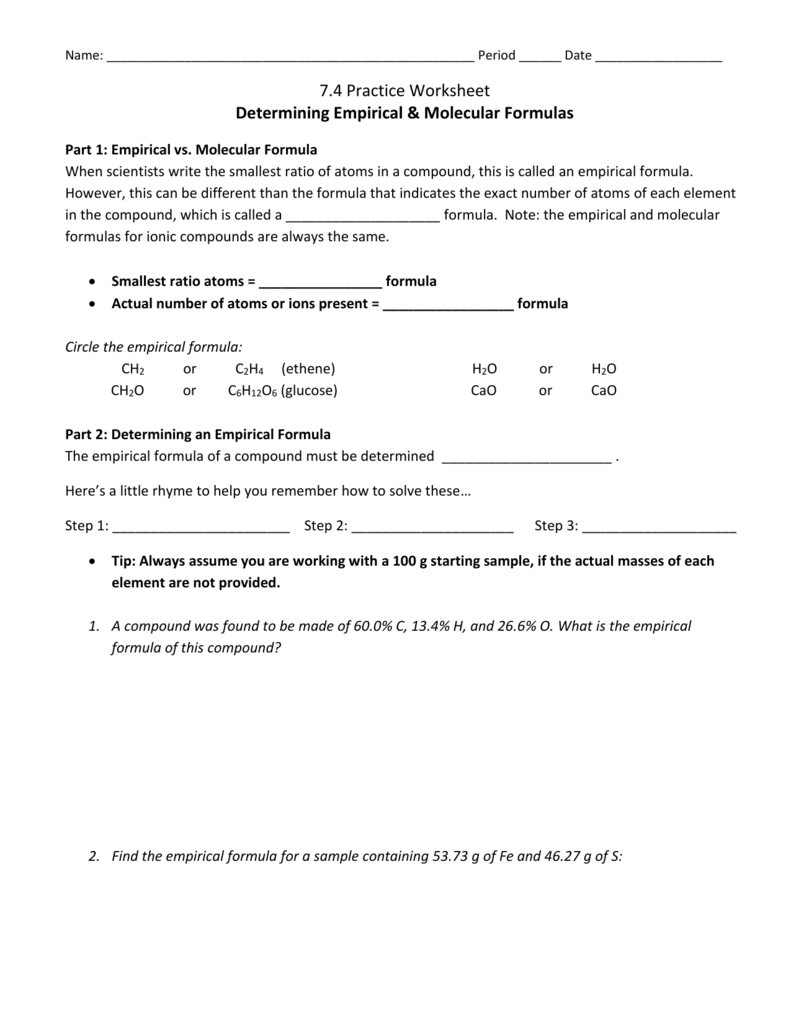 Determining Empirical Molecular Formulas
Determining Empirical Molecular Formulas
 Determining Molecular Formulas Ck 12 Foundation
Determining Molecular Formulas Ck 12 Foundation
Empirical Formula And Molecular Formula Calculations For Mcat
 Empirical And Molecular Formula Notes
Empirical And Molecular Formula Notes
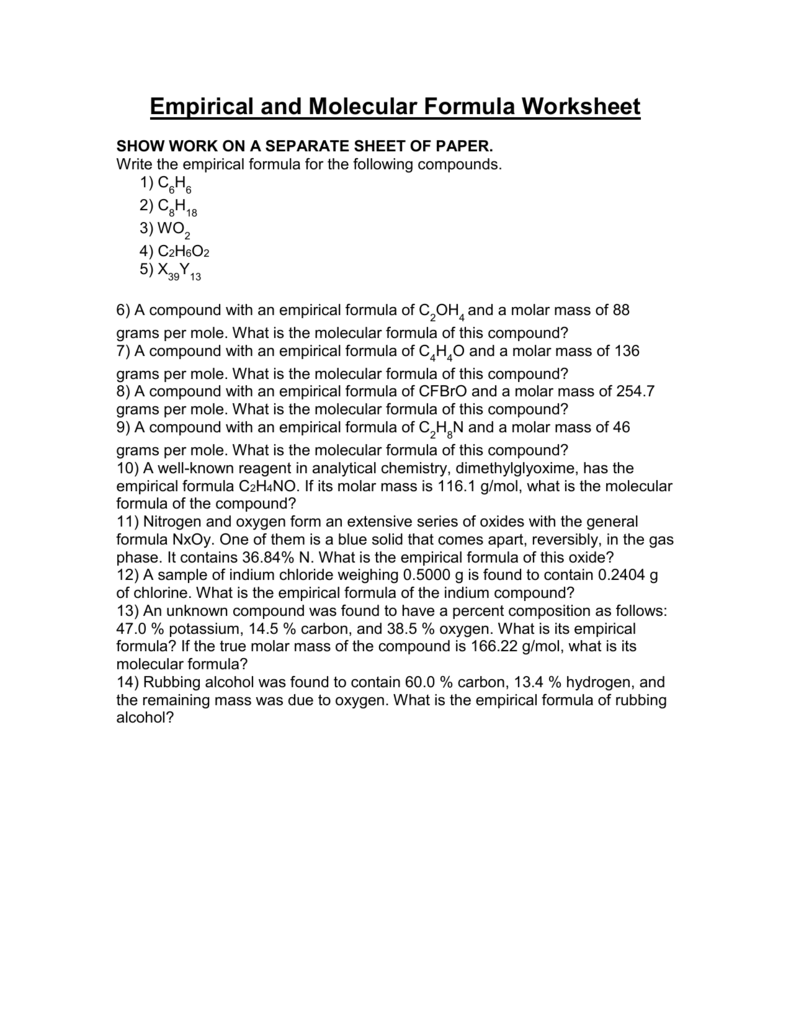 Empirical And Molecular Formula Worksheet
Empirical And Molecular Formula Worksheet
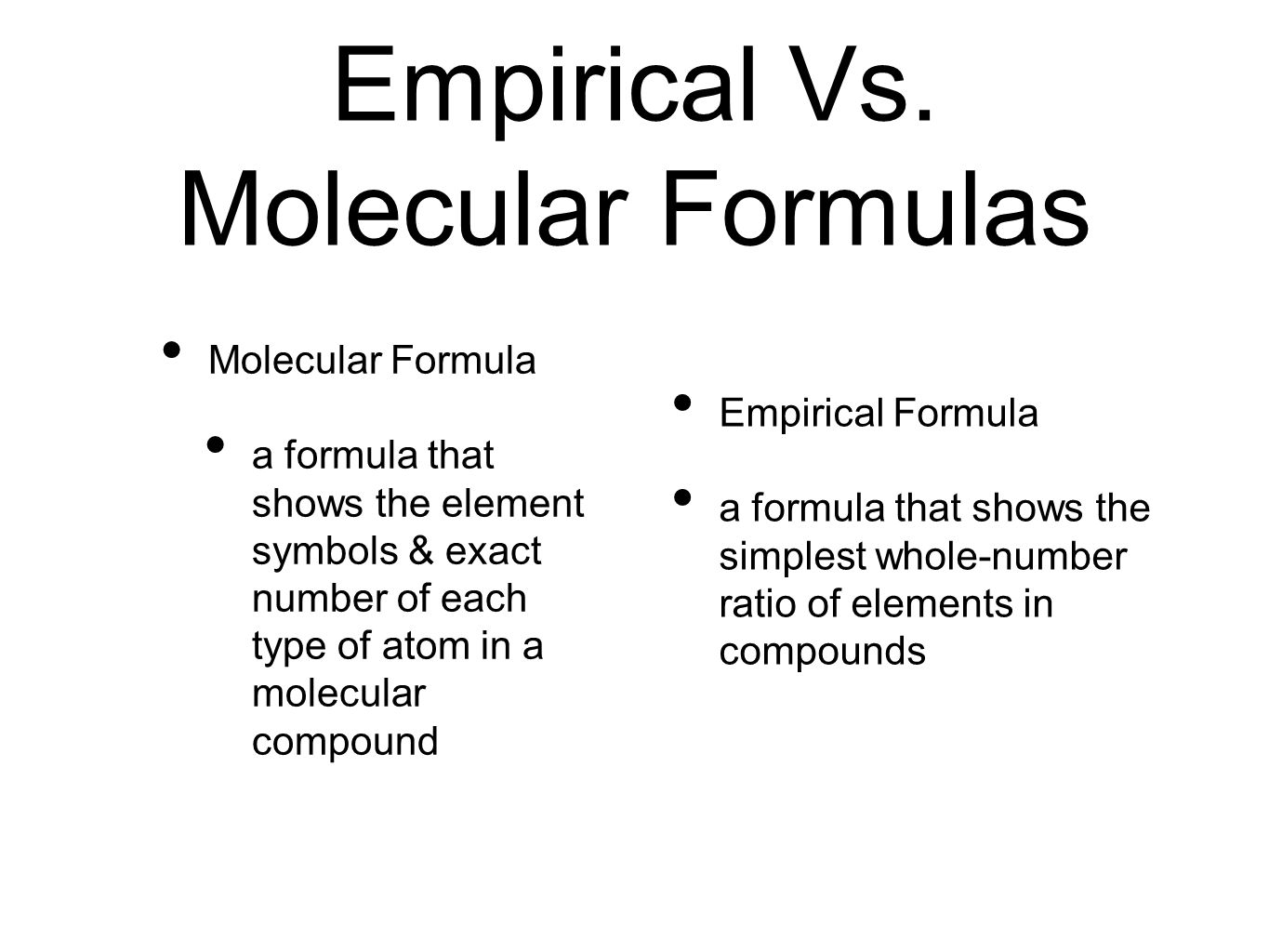 Empirical Formula Molecular Formula Ppt Download
Empirical Formula Molecular Formula Ppt Download
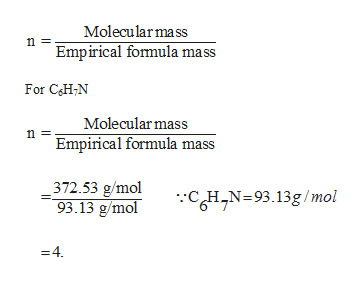 Answered From The Given Empirical Formula And Bartleby
Answered From The Given Empirical Formula And Bartleby
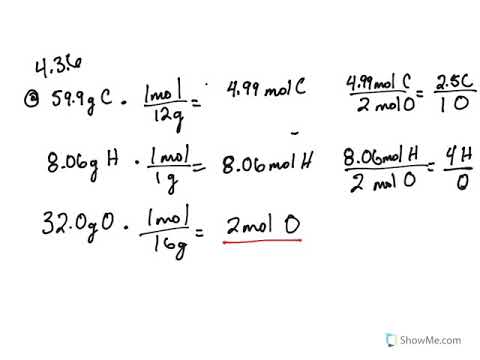 4 3 Empirical And Molecular Formulas Problems Chemistry
4 3 Empirical And Molecular Formulas Problems Chemistry
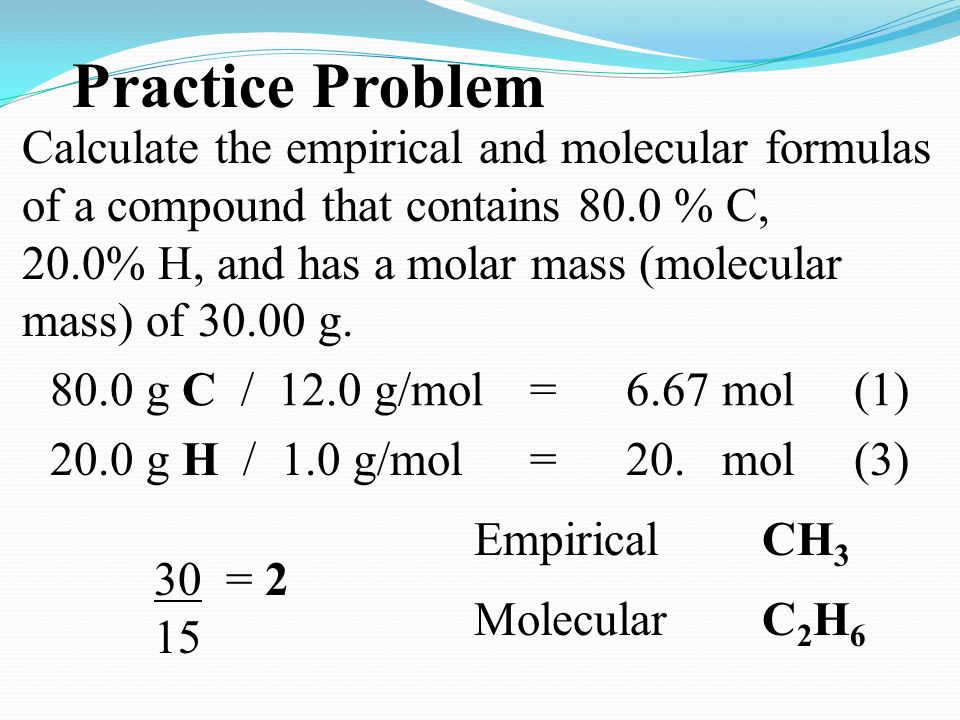 Empirical Formula Molecular Formula Ppt Video Online Download
Empirical Formula Molecular Formula Ppt Video Online Download
 Ppt Empirical And Molecular Formula Notes Powerpoint
Ppt Empirical And Molecular Formula Notes Powerpoint
 Empirical And Molecular Formula Practice Problems
Empirical And Molecular Formula Practice Problems

 Determining Empirical And Molecular Formulas Chemistry Tutorial
Determining Empirical And Molecular Formulas Chemistry Tutorial
 Chemistry Mysteries Empirical And Molecular Formulas
Chemistry Mysteries Empirical And Molecular Formulas
 Ppt Empirical Molecular Formulas Powerpoint Presentation Free
Ppt Empirical Molecular Formulas Powerpoint Presentation Free
What Is An Empirical Formula Quora
 Empirical And Molecular Formulas Of Compounds Mole And Empirical
Empirical And Molecular Formulas Of Compounds Mole And Empirical
1 Find The Formula Mass Of C3h5o2 3 12 01 G 5 1 01 2 16 00
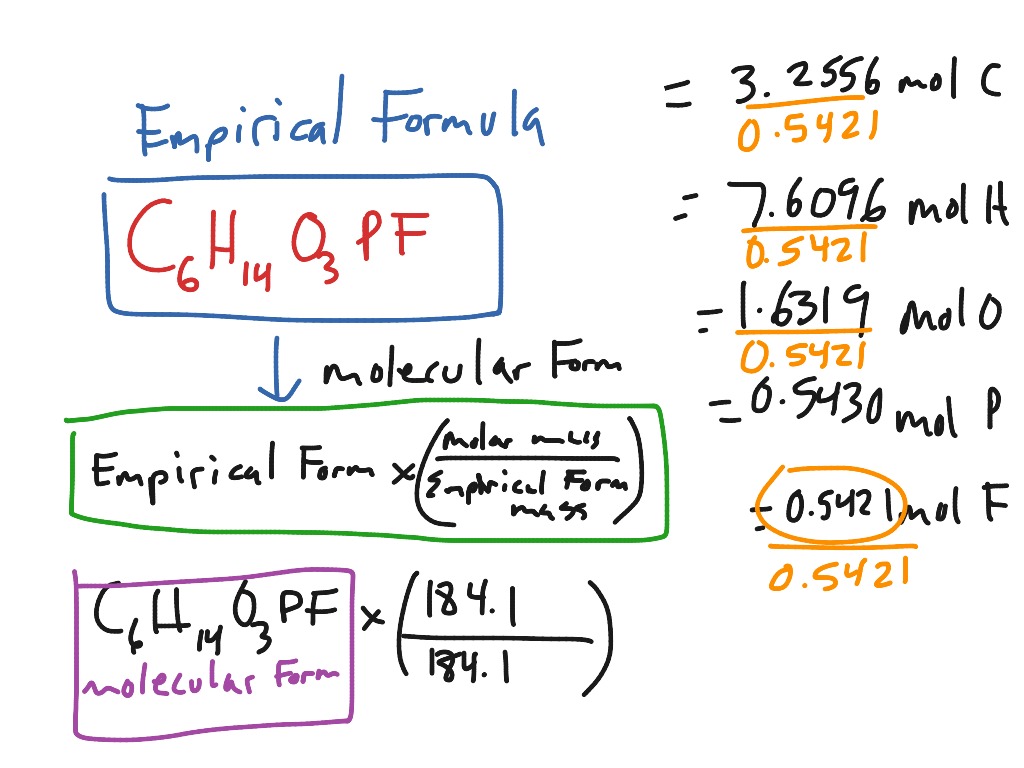 Empirical And Molecular Formulas 6 Science Chemistry Empirical
Empirical And Molecular Formulas 6 Science Chemistry Empirical
/Molecular-formula-58e51cdc3df78c5162a9e340.jpg) Calculate Empirical And Molecular Formulas
Calculate Empirical And Molecular Formulas
 Worksheet Empirical And Molecular Formulas
Worksheet Empirical And Molecular Formulas
Section 6 5 Emperical Versus Molecular Formulas
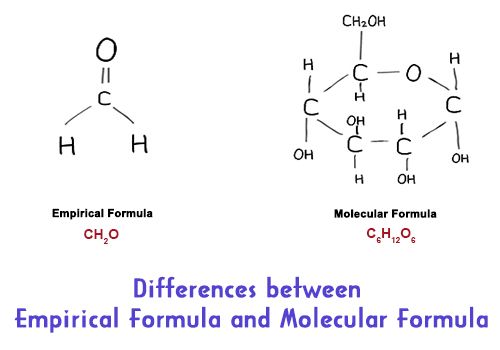 Differences Between Empirical Formula And Molecular Formula Qs Study
Differences Between Empirical Formula And Molecular Formula Qs Study
 Determining Empirical And Molecular Formulas Chemistry Tutorial
Determining Empirical And Molecular Formulas Chemistry Tutorial
Percent Composition And Empirical And Molecular Formulas Chemistry
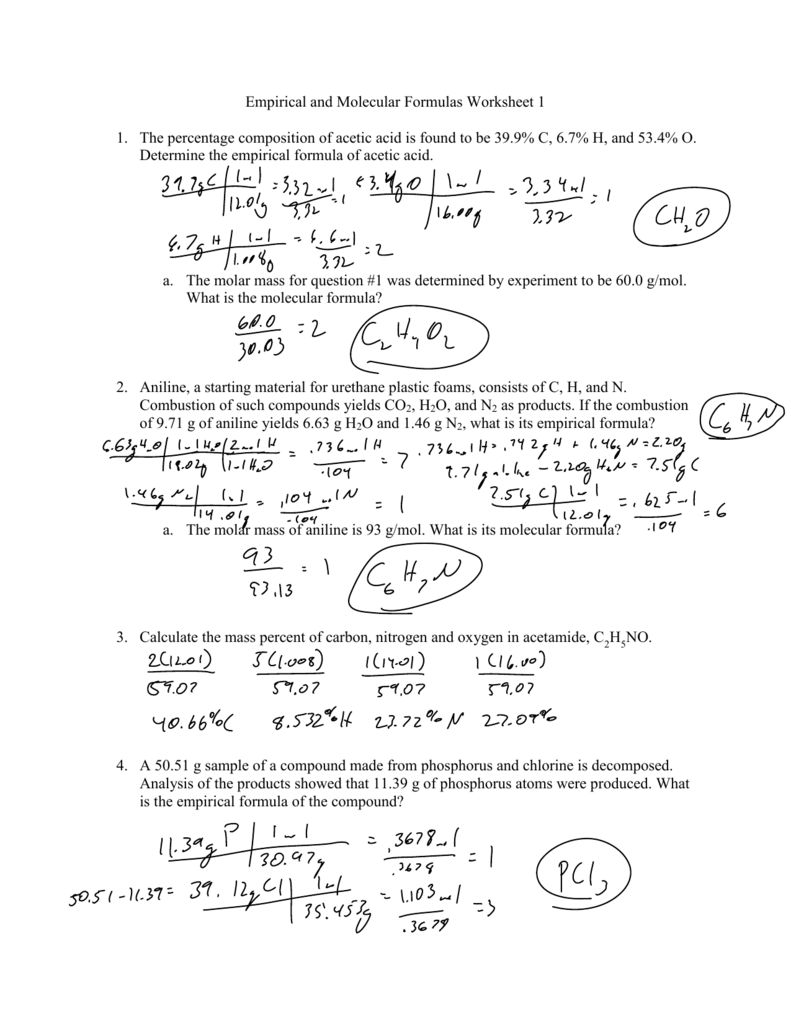 Empirical And Molecular Formulas Worksheet 1 1 The Percentage
Empirical And Molecular Formulas Worksheet 1 1 The Percentage
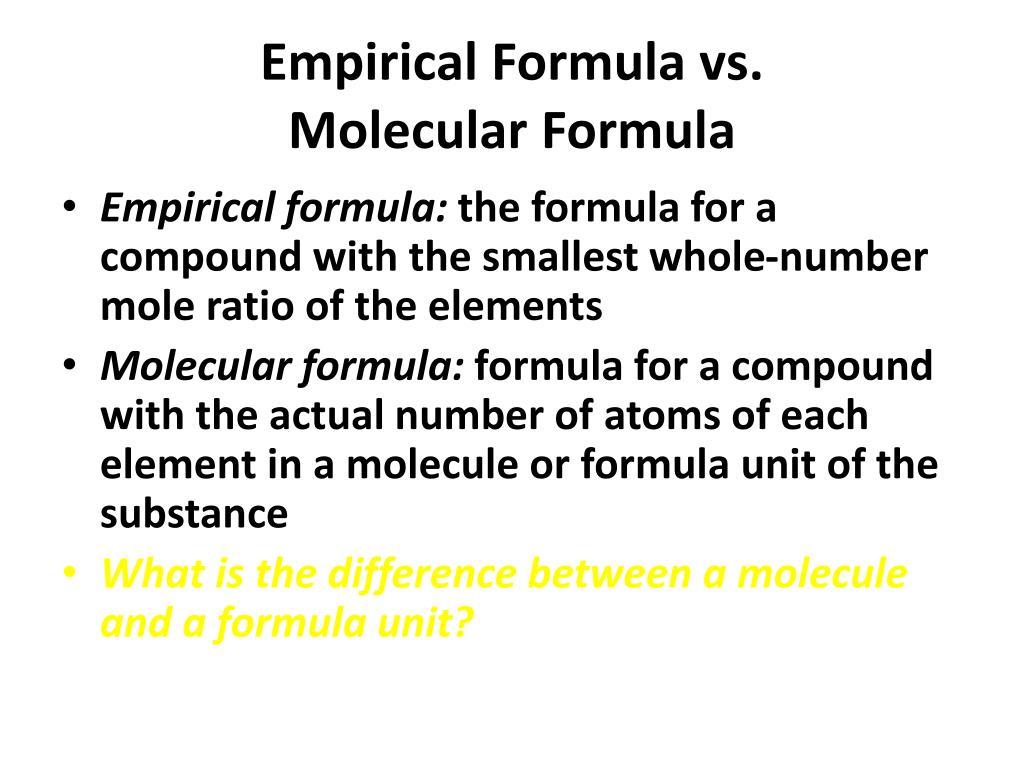 Ppt Empirical Formula Vs Molecular Formula Powerpoint
Ppt Empirical Formula Vs Molecular Formula Powerpoint
 5 5 Molecular Formula Percent Composition Review Of Empirical
5 5 Molecular Formula Percent Composition Review Of Empirical
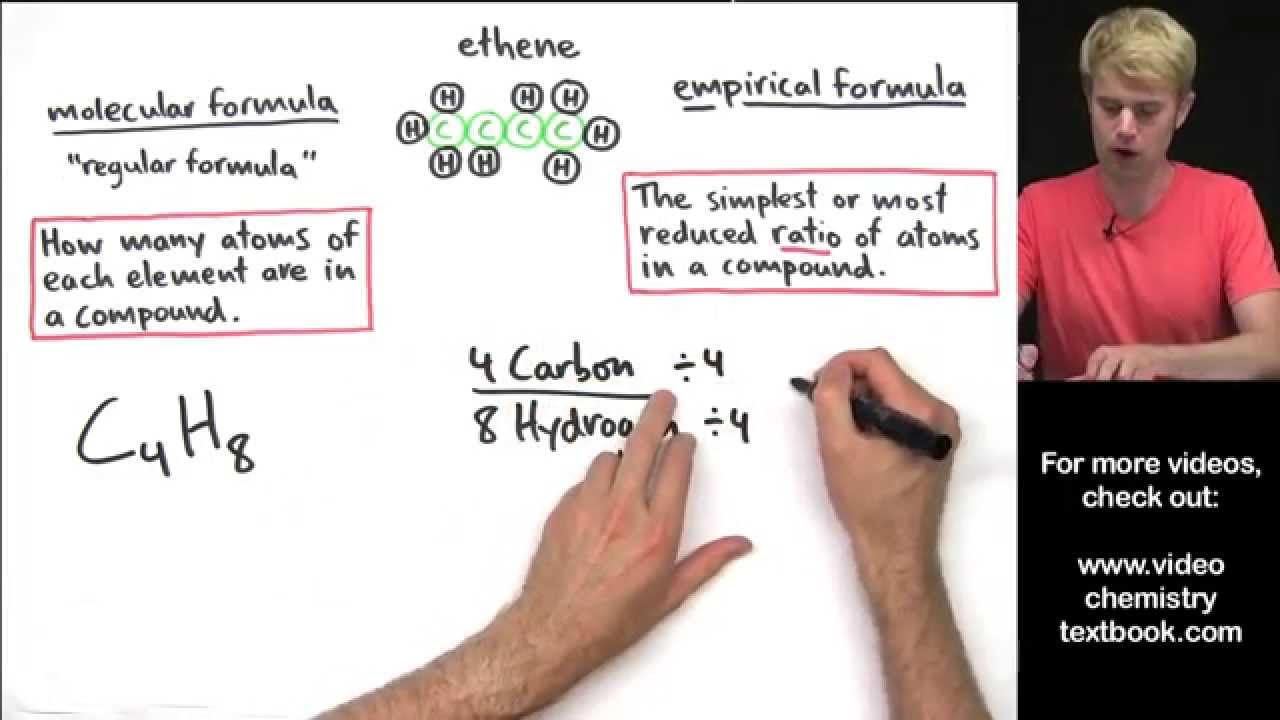 Empirical Formula And Molecular Formula Introduction Youtube
Empirical Formula And Molecular Formula Introduction Youtube

 What Is The Empirical Formula Of Melamine Socratic
What Is The Empirical Formula Of Melamine Socratic
Determining Empirical And Molecular Formulas Lessons Tes Teach
 Empirical Molecular Formula Practice
Empirical Molecular Formula Practice
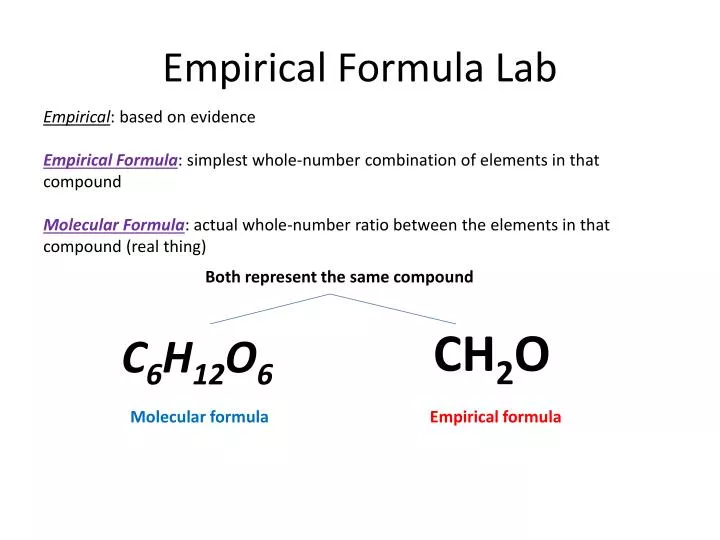 Ppt Empirical Formula Lab Powerpoint Presentation Free Download
Ppt Empirical Formula Lab Powerpoint Presentation Free Download
 Fillable Online Acschools Empirical And Molecular Formula
Fillable Online Acschools Empirical And Molecular Formula
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsyxqgzrpsxomf6woq Dnja Pa0z3vzmqs Pqiqil7owmk7 Y O Usqp Cau


Posting Komentar
Posting Komentar