What Is A Spectator Ion Quizlet
These ions are called spectator ions since they don t participate in the chemical reaction at all they just watch. In the net ionic equation all species with s l and g will be unchanged.
 Chemistry Chemical Reactions Notes Flashcards Quizlet
Chemistry Chemical Reactions Notes Flashcards Quizlet
A spectator ion is an ion that is equally present on both sides of the reaction.
What is a spectator ion quizlet. A net ionic equation includes only those ions or compounds that undergo chemical change. A chemical equation written without the spectator ions is called a net ionic equation. Learn spectator ions with free interactive flashcards.
Learn vocabulary terms and more with flashcards games and other study tools. Choose from 31 different sets of spectator ions flashcards on quizlet. A spectator ion is one that exists in the same form on both the reactant and product sides of a chemical reaction.
Any aq that remain on both sides of the equation reactants and products can be canceled out. K2s fe no3 2 fes s kno3 aq fe2 aq s2 aq fes s give the spectator ions for the reaction that occurs when aqueous solutions k2s and fe no3 2 are mixed. An ion that is not directly involved in a chemical reaction.
An ion that does not change oxidation number or composition during a reaction. 2 na co 2 3 aq cu 2 aq so 2 4 aq 2 na aq so 2 4 aq cuco 3. Start studying common spectator ions.
A spectator ion can therefore be observed in the reaction of aqueous solutions of sodium carbonate and copper ii sulfate but does not affect the equilibrium. Spectator ion definition spectator ions may be either cations positively charged ions or anions negatively charged ions. The na and so 2.
A spectator ion is an ion that exists as a reactant and a product in a chemical equation. An equation for a reaction in solution showing only those particles that are directly involved in the chemical change. Give the net ionic equation for the reaction if any that occurs when aqueous solutions of k2s and fe no3 2 are mixed.
They do not undergo any chemical change at all. These are called spectator ions and they don t participate in the reaction. In order to figure this out we first have to balance the.
 Chemistry 13 4 Precipitation Reactions And More Flashcards Quizlet
Chemistry 13 4 Precipitation Reactions And More Flashcards Quizlet
Https Www Ranchorams Org Ourpages Auto 2017 4 17 53013028 Chem 20community 20review 20powerpoint 20 Pdf
 Changes In Matter Involve The Rearrangement And Or Reorganizations
Changes In Matter Involve The Rearrangement And Or Reorganizations

 History Of Media Communication Midterm Nyu Studocu
History Of Media Communication Midterm Nyu Studocu
 Reactions In Aqueous Solutions Flashcards Quizlet
Reactions In Aqueous Solutions Flashcards Quizlet
 Chem 60 Chapter 7 Chemical Reactions Flashcards Quizlet
Chem 60 Chapter 7 Chemical Reactions Flashcards Quizlet
 What Is The Net Ionic Equation Of Reaction Mgso4 With Ba No3 2
What Is The Net Ionic Equation Of Reaction Mgso4 With Ba No3 2
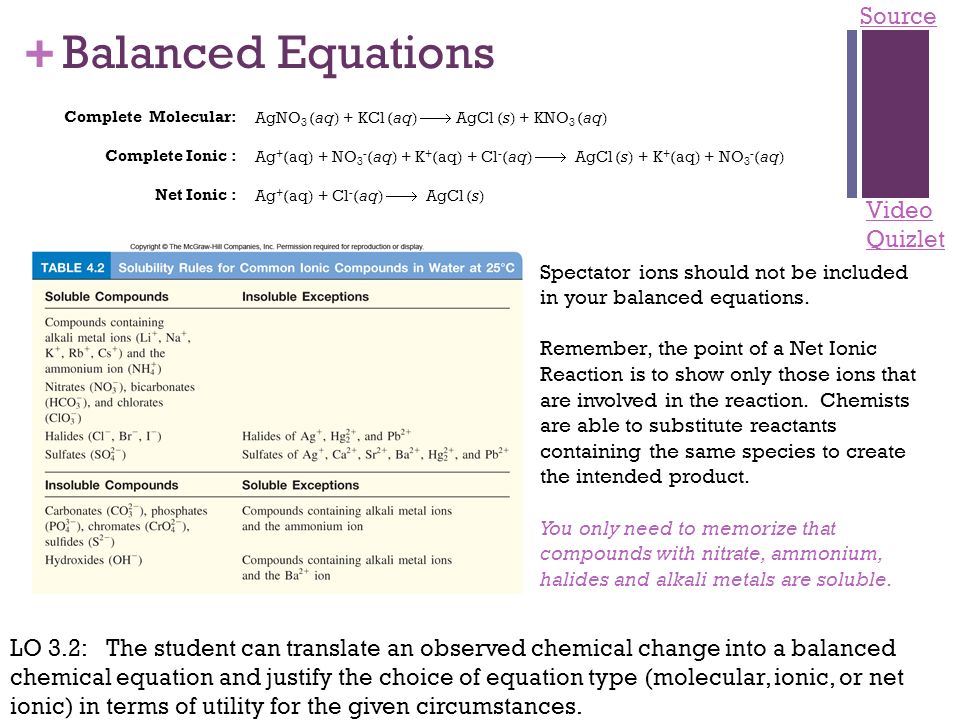
 Ap Chemistry Exam Review Powerpoint Presentation Free Online
Ap Chemistry Exam Review Powerpoint Presentation Free Online
 T D 301 Study Guide Fall 2015 Midterm Zeami Motokiyo Anton
T D 301 Study Guide Fall 2015 Midterm Zeami Motokiyo Anton
 Theatre 210 Final Study Guide Winter 2018 Cal Poly Studocu
Theatre 210 Final Study Guide Winter 2018 Cal Poly Studocu
 Chemistry Formulas Flashcards Quizlet
Chemistry Formulas Flashcards Quizlet
Https Www Ranchorams Org Ourpages Auto 2017 4 17 53013028 Chem 20community 20review 20powerpoint 20 Pdf
 Chemistry Unit 13 Flashcards Quizlet
Chemistry Unit 13 Flashcards Quizlet
 Chemistry Chapter 11 Chemical Reactions Flashcards Quizlet
Chemistry Chapter 11 Chemical Reactions Flashcards Quizlet
 Solved In Many Chemical Reactions Of Two Or More Compound
Solved In Many Chemical Reactions Of Two Or More Compound
 Chem 9 2 To 9 3 Diagram Quizlet
Chem 9 2 To 9 3 Diagram Quizlet
 What Is The Net Ionic Equation Of Reaction Becl2 With Naoh
What Is The Net Ionic Equation Of Reaction Becl2 With Naoh
 Chapter 9 Chemical Reactions And Equations Flashcards Quizlet
Chapter 9 Chemical Reactions And Equations Flashcards Quizlet
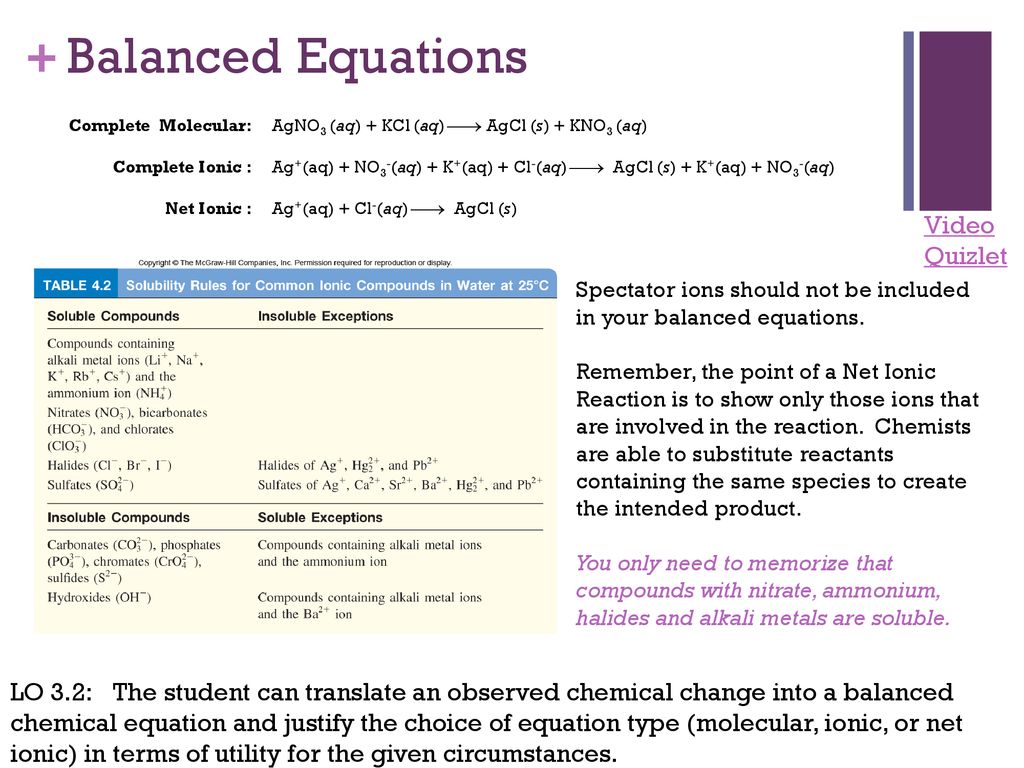
 Ap Chemistry Exam Review Ppt Download
Ap Chemistry Exam Review Ppt Download
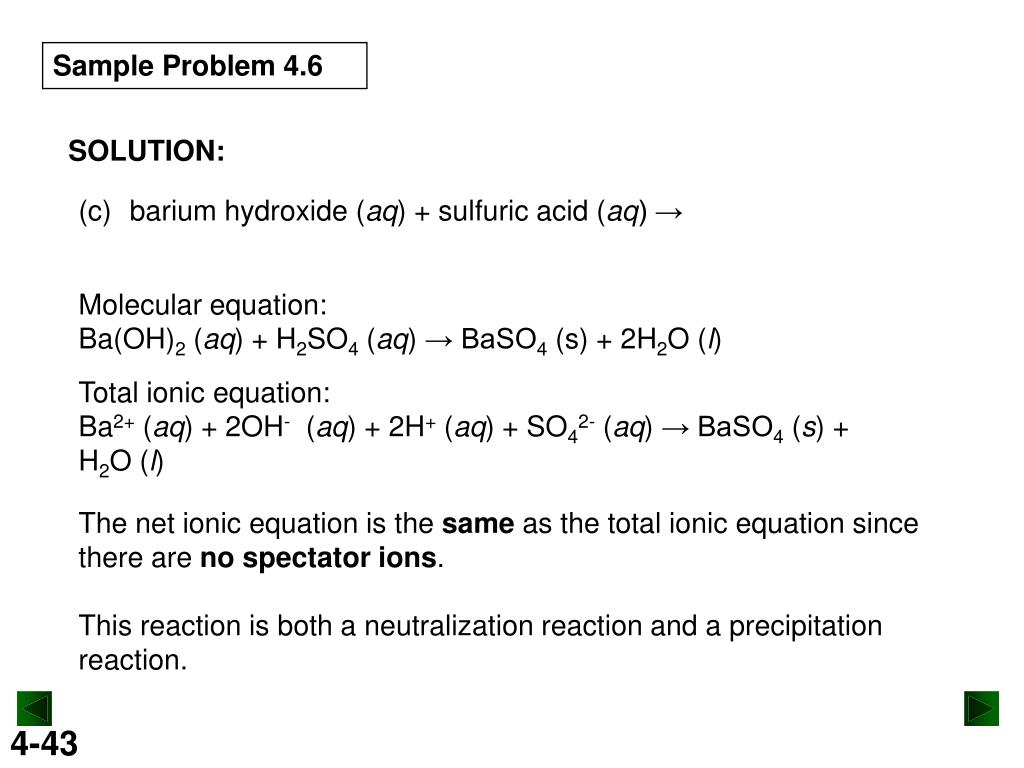
 Ap Chemistry Exam Review Supertallteacher Powerpoint
Ap Chemistry Exam Review Supertallteacher Powerpoint
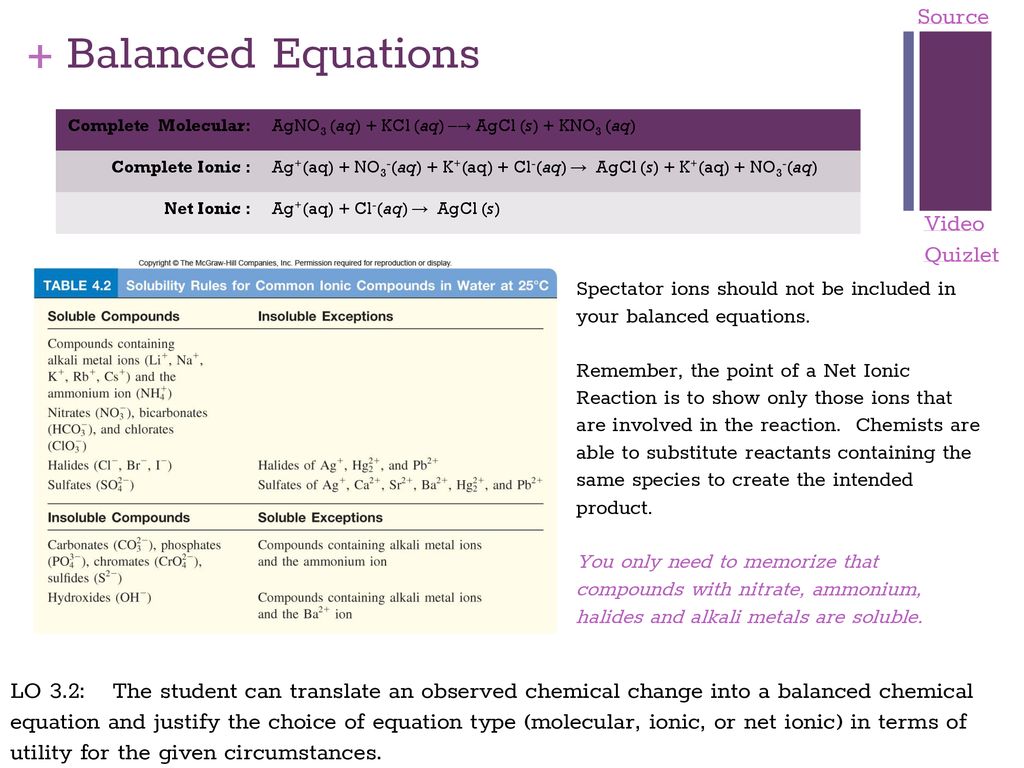 Big Idea 3 Chemical Reactions Ppt Download
Big Idea 3 Chemical Reactions Ppt Download
 Chemistry Ch 11 3 Vocab Jen Alfon Flashcards Quizlet
Chemistry Ch 11 3 Vocab Jen Alfon Flashcards Quizlet
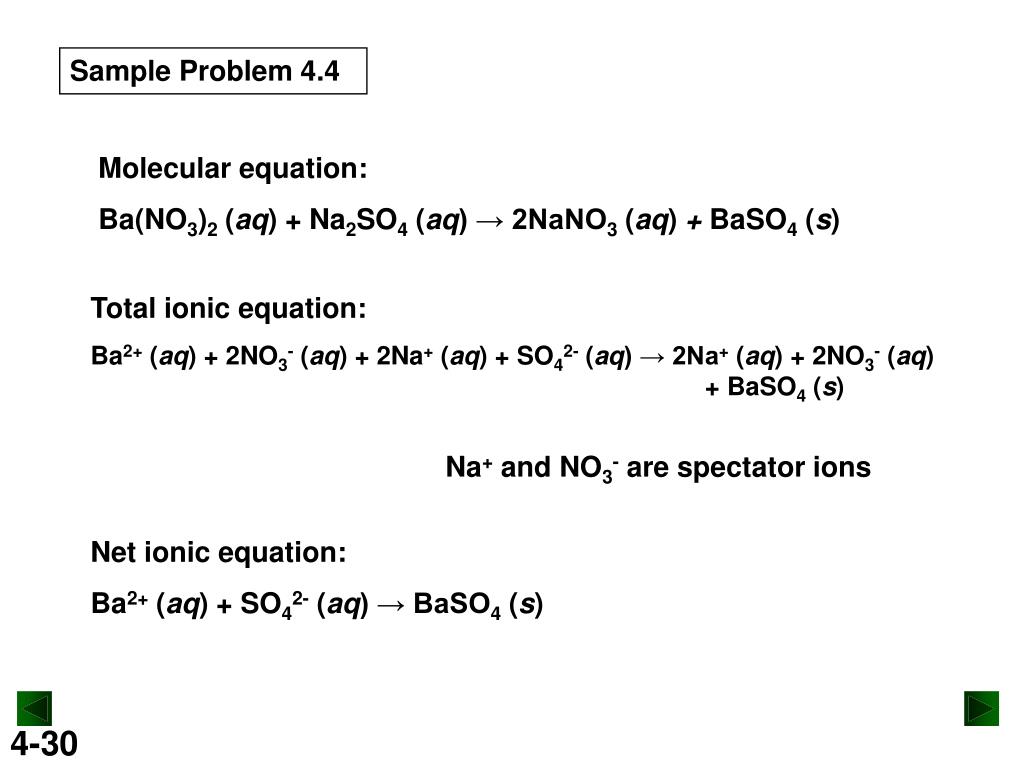 What Is The Net Ionic Equation Of Reaction Mgso4 With Ba No3 2
What Is The Net Ionic Equation Of Reaction Mgso4 With Ba No3 2
 Exam 2 Chemistry Flashcards Quizlet
Exam 2 Chemistry Flashcards Quizlet
 What Is The Net Ionic Equation Of The Reaction Of Fecl2 With
What Is The Net Ionic Equation Of The Reaction Of Fecl2 With

 Iodide Persulphate Reaction Flashcards Quizlet
Iodide Persulphate Reaction Flashcards Quizlet






Posting Komentar
Posting Komentar